Why the COVID-19 Delta Variant Spreads So Easily and Infects People So Quickly
0 View
Share this Video
- Publish Date:
- 8 November, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
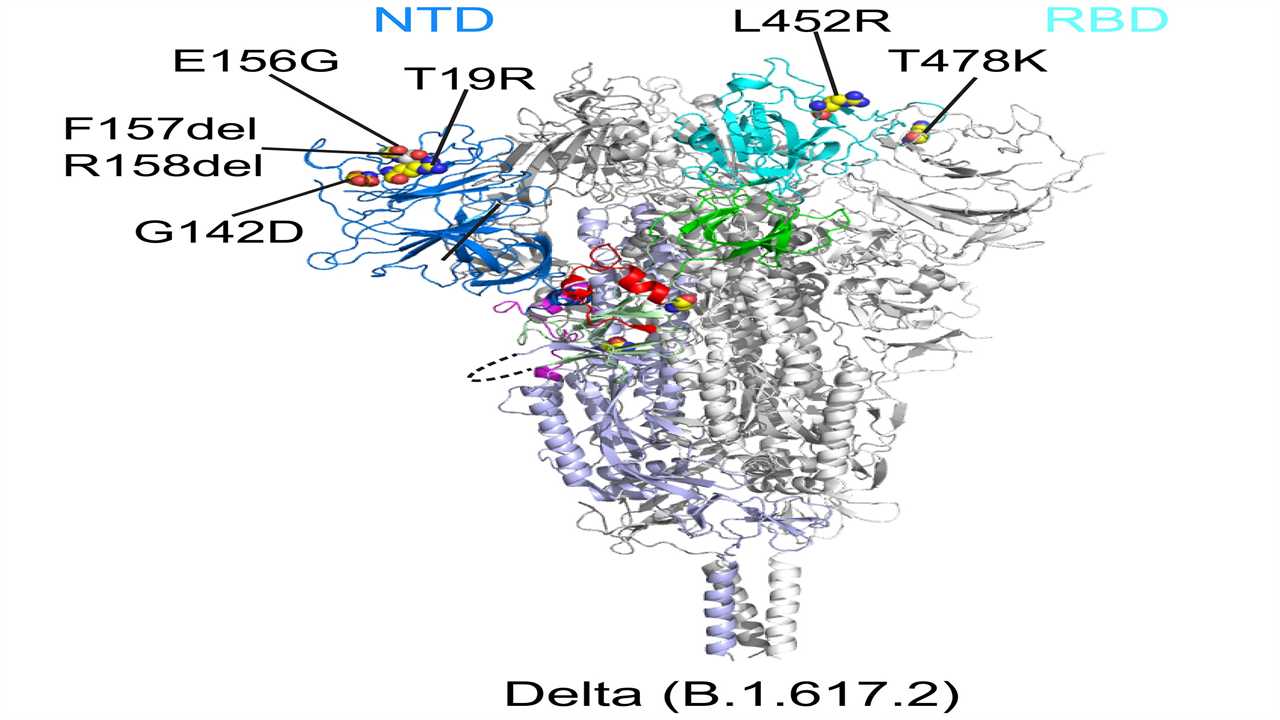
This ribbon diagram shows the structure of the spike protein of the Delta variant before the virus fuses with its target cell. The N-terminal domain (NTD) is shown in blue and the receptor binding domain (RBD) in cyan. Credit: Bing Chen, PhD, Boston Children’s Hospital
The findings have implications for the next generation of COVID-19 vaccines and treatments.
The Delta variant of SARS-CoV-2 has conquered the world and has become the dominant variant within a few months. A new Boston Children’s Hospital study, published Oct. 26, 2021 in Science, explains why Delta spreads so easily and infects people so quickly, and suggests a more targeted strategy for developing future COVID-19 vaccines and treatments. .
Last spring, study leader Bing Chen, PhD, showed how several earlier SARS-CoV-2 variants (alpha, beta, G614) became more contagious than the original virus. Each variant received a genetic change that stabilized the spike protein – the surface protein on which current vaccines are based. This mutation increased the variant’s ability to get into cells.
The Delta variant, which arose shortly afterwards, is the most contagious variant known to date. Chen and colleague wanted to understand why. “We thought something completely different had to be done because Delta stands out among all the variants,” Chen says. “We found a property that we believe explains the transferability and appears to be unique to Delta so far.”
Fast fusion, fast import
For SARS-CoV-2 to infect our cells, the spikes first bind to a receptor called ACE2. The spikes then drastically change shape and fold in on themselves. This clipping motion fuses the outer membrane of the virus with the membranes of our cells.
Using two types of cell-based assays, Chen and colleagues show that Delta’s spike protein is especially adept at membrane fusion. This allowed a simulated Delta virus to infect human cells much faster and more efficiently than the other five SARS-CoV-2 variants (see bar graph). That was especially true when cells had relatively low amounts of the ACE2 receptor.
The Delta variant of SARS-CoV-2 fused with cell membranes much faster than five other variants (Alpha, Beta, G614, Gamma and Kappa). Credit: Zhang J; et al. Science 2021 Oct 26; DOI: 10.1126/science.abl9463
“Membrane fusion requires a lot of energy and needs a catalyst,” Chen explains. “Of the different variants, Delta stood out for its ability to catalyze membrane fusion. This explains why Delta is transmitted much faster, why you can get it after a shorter exposure, and why it can infect more cells and produce such high viral loads in the body.”
Designing interventions, informed by structure
To learn how mutations in the variants affect the structure of the spike protein, Chen and colleagues used cryoelectron microscopy, which has resolution down to the atomic level. They imaged spike proteins from the Delta, Kappa and Gamma variants and compared them to spikes from the previously characterized G614, Alpha and Beta variants.
All variants had changes in two key parts of the spike protein recognized by our immune system’s neutralizing antibodies: the receptor binding domain (RBD), which binds to the ACE2 receptor, and the N-terminal domain (NTD). Mutations in both domains may reduce the ability of neutralizing antibodies to bind to the peak.
“The first thing we noticed about Delta was that there was a big change in the NTD, which is responsible for the resistance to neutralizing antibodies,” Chen says. “The RBD also changed, but this led to little change in antibody resistance. Delta still remained sensitive to all of the RBD-targeted antibodies we tested.”
Looking at the other variants, the researchers found that each modified the NTD in different ways that changed its contours. The RBD was also mutated, but the changes were more limited. Overall, the structure of the RBD remained relatively stable across the variants, presumably preserving its critical role in binding to the ACE2 receptor. The researchers therefore believe that the RBD is a more favorable target for next-generation vaccines and antibody treatments.
“We wouldn’t want to focus on the NTD because the virus can mutate and change structure very quickly; it’s a moving target,” Chen explains. “It may be most effective to target the RBD — to focus the immune system on that critical domain rather than the entire spike protein.”
Reference: “Membrane fusion and immune evasion by the spike protein of the SARS-CoV-2 Delta variant” by Jun Zhang, Tianshu Xiao, Yongfei Cai, Christy L. Lavine, Hanqin Peng, Haisun Zhu, Krishna Anand, Pei Tong, Avneesh Gautam, Megan L. Mayer, Richard M. Walsh, Jr., Sophia Rits-Volloch, Duane R. Wesemann, Wei Yang, Michael S. Seaman, Jianming Lu, and Bing Chen, Oct. 26, 2021, Science.
DOI: 10.1126/science.abl9463
Jun Zhang, PhD, and Tianshu Xiao, PhD, of Boston Children’s Hospital were co-first authors on the paper. The study was funded by Emergent Ventures, the Massachusetts Consortium on Pathogen Readiness (MassCPR), and the National Institutes of Health (grants AI147884, AI141002, AI127193, AI39538, and AI165072).










