Treatments Against the Coronavirus’s Tangled Strands of RNA
0 View
Share this Video
- Publish Date:
- 26 November, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
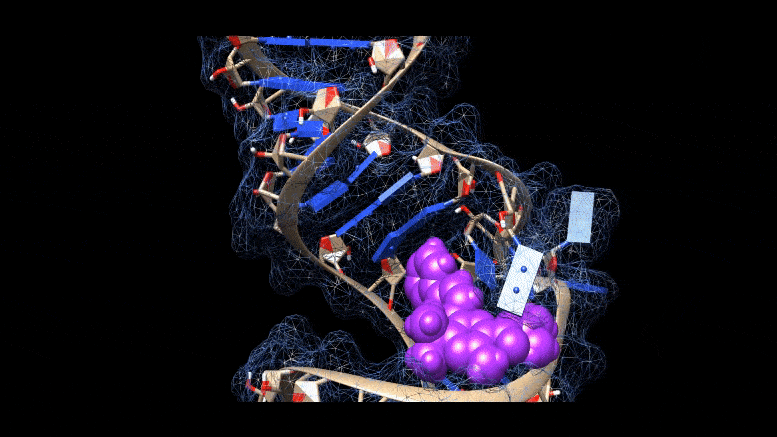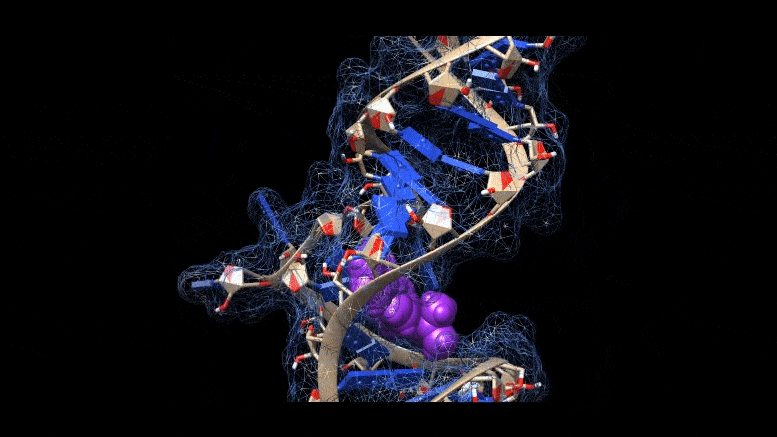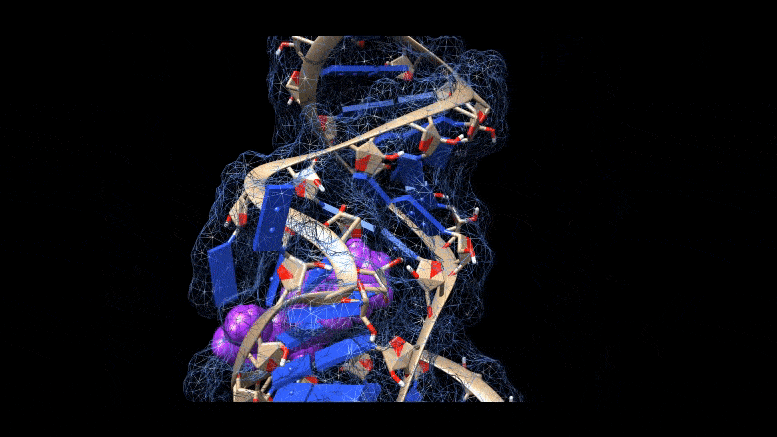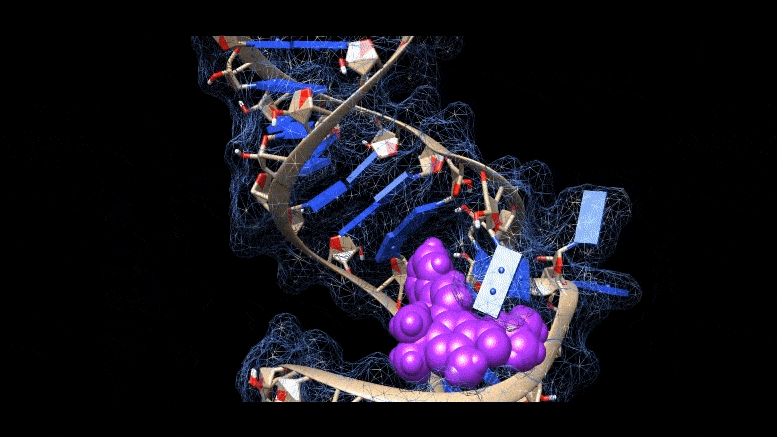
Researchers are working on new ways to cure COVID-19 infections, using molecules that bind to three-dimensional folds in the virus’s genetic material. Credit: Martina Zafferani, Duke University
The tangled strands of RNA from the coronavirus may offer new ways to treat people who become infected.
To the untrained eye, the loops, kinks and folds in the single strand of RNA that make up the coronavirus genome look like a tangle of spaghetti or tangled yarn. But for researchers like Amanda Hargrove, a chemistry professor at Duke University, the complex shapes RNA takes as it folds could have untapped therapeutic potential in the fight against COVID-19.
In a study published today (November 26, 2021) in the journal Science Advances, Hargrove and colleagues identified chemical compounds that can attach to these 3-D structures and block the virus’ ability to replicate.
“These are the first molecules with antiviral activity that specifically target the RNA of the virus, so it’s a completely new mechanism in that sense,” Hargrove said.
Even more than 18 months into the pandemic, that’s good news. We have vaccines to prevent COVID-19, but effective, easy-to-administer drugs to help people survive and recover once infected remain limited.
In some parts of the world, the virus is on the decline, but in others where vaccines are scarce, cases are still on the rise. And even in regions with easy access to vaccines, the reluctance of COVID-19 vaccines means many of the world’s eight billion people remain vulnerable to infection.
To infect your cells, the coronavirus must break in, deliver its genetic instructions in the form of RNA, and hijack the body’s molecular machinery to make new copies of itself. The infected cell becomes a virus factory, reads the 30,000 nucleotide “letters” of the virus’s genetic code and produces the proteins the virus needs to multiply and spread.
Most antivirals — including remdesivir, molnupiravir and Paxlovid, the only antiviral drugs for COVID-19 approved or eligible for approval by the FDA — work by binding to these proteins. But Hargrove and colleagues take a different approach. They have identified the first molecules that target the viral genome itself — and not just the linear sequence of A’s, C’s, G’s and U’s, but the complex three-dimensional structures into which the RNA strand folds.
When the first terrifying hints of the pandemic began to make headlines, the team, including Hargrove, Blanton Tolbert of Case Western Reserve University, and Gary Brewer and Mei-Ling Li of Rutgers, was already researching potential drug candidates for fighting another RNA virus – Enterovirus 71, a common cause of hand, foot and mouth disease in children.
They had identified a class of small molecules called amilorides that can bind to hairpin-like folds in the virus’s genetic material and throw a key into the virus’s replication.
To see if the same compounds could work against coronaviruses, they first tested 23 amiloride-based molecules against another much less deadly coronavirus responsible for many common colds. They identified three compounds that, when added to infected monkey cells, reduced the amount of virus within 24 hours of infection without causing collateral damage to their host cells. They also showed greater effects at higher doses. The researchers got similar results when they tested the molecules on cells infected with SARS-CoV-2, the virus that causes COVID-19.
Further work showed that the molecules stopped the virus from building up by binding to a site in the first 800 letters of the viral genome. Most of this piece of RNA does not itself code for proteins, but directs their production.
The area folds in on itself, forming multiple bulges and hairpin-like structures. Using computer modeling and a technique called nuclear magnetic resonance spectroscopy, the researchers were able to analyze these 3D RNA structures and determine where the chemical compounds were binding.
The researchers are still trying to figure out exactly how these compounds stop the virus from multiplying once they’re bound to the genome.
When it comes to using RNA as a drug target, Hargrove says the field is still in its infancy. Part of the reason is that RNA structures are unstable. They bounce around a lot more than their protein counterparts, making it difficult to design molecules that can interact with them in specific ways.
“The tie bag you’re looking for isn’t usually even there,” Hargrove said.
In addition, 85% of the RNA in an infected cell does not belong to the virus, but to the ribosomes — cellular particles made of RNA and protein — of its human host. “There’s a sea of competition,” Hargrove said.
But Hargrove is hopeful. The first small-molecule drug that works by binding directly to non-ribosomal RNA, rather than proteins, was approved by the FDA last August to treat people with a devastating disease called spinal muscular atrophy. “So while there are a lot of challenges, it’s not impossible,” Hargrove said.
The researchers have applied for a patent for their method. They want to modify the compounds to make them more potent, then test them in mice “to see if this could be a viable drug candidate,” Hargrove said.
This isn’t the first time coronaviruses have sparked an outbreak, and it probably won’t be the last, the researchers say. Over the past two decades, the same family of viruses has been responsible for SARS, which emerged in China and spread to more than two dozen countries in 2002, and MERS, which was first reported in Saudi Arabia in 2012.
The researchers determined that the loops and bulges of RNA they identified have remained essentially unchanged through evolution across related coronaviruses in bats, rats and humans, including those that caused the SARS and MERS outbreaks. That means their method could potentially fight more than just SARS-CoV-2, the virus that causes COVID-19.
Obviously, more antivirals would be valuable weapons, so when the next pandemic hits, we’ll be better prepared. Having more drugs on hand would have another benefit: fighting resistance. Viruses mutate over time. If drugs with different mechanisms of action can be combined, it becomes less likely that the virus will develop resistance to all of them at the same time and become impossible to treat, Hargrove said.
“This is a new way of thinking about antivirals for RNA viruses,” Hargrove said.
Reference: “Amilorides inhibit SARS-CoV-2 replication in vitro by targeting RNA structures” by Martina Zafferani, Christina Haddad, Le Luo, Jesse Davila-Calderon, Liang Yuan-Chiu, Christian Shema Mugisha, Adeline Monaghan, Andrew Kennedy, Joseph Yesselman, Robert Gifford, Andrew Tai, Sebla Kutluay, Mei-Ling Li, Gary Brewer, Blanton Tolbert, and Amanda Hargrove, Nov. 26, 2021, Science Advances.
DOI: 10.1126 / sciaadv.abl6096
The researchers collaborated with seven institutions for this study, including Rutgers University, Case Western Reserve University, Washington University School of Medicine in St. Louis, University of Nebraska-Lincoln, University of Glasgow and the University of Michigan.
This research was supported by the National Institute of General Medical Sciences (R35GM124785, GM126833), Tobacco Settlement Fund (21-5734-0010), Medical Research Council of the United Kingdom (MC_UU_12014/12), and Duke University.