Structural Changes Identified in COVID Alpha and Beta Variants – Suggests Need for Updated Vaccine Booster
0 View
Share this Video
- Publish Date:
- 27 June, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
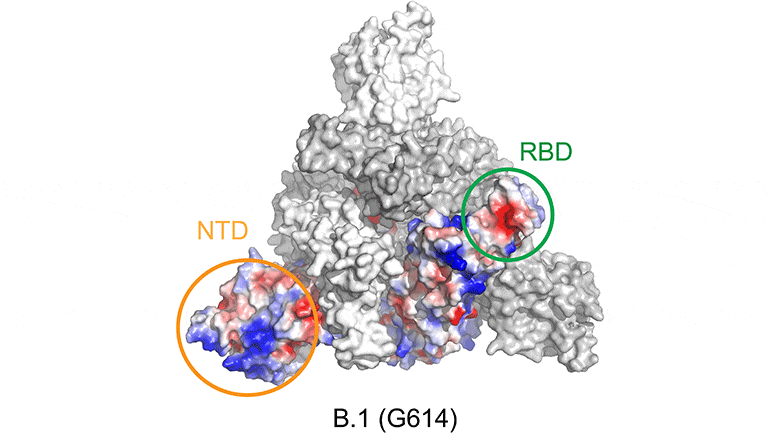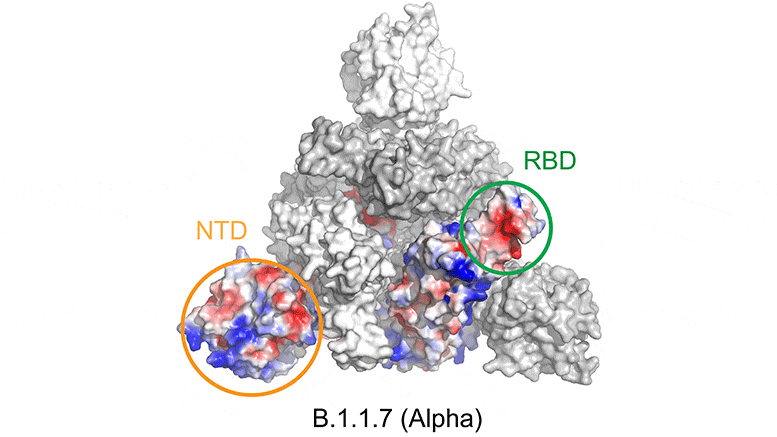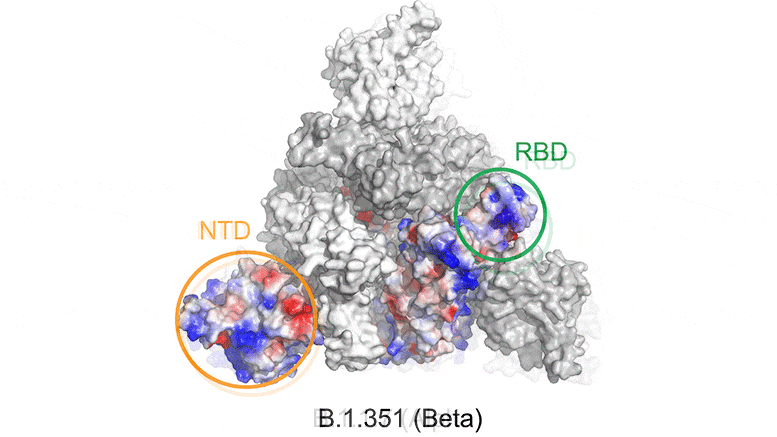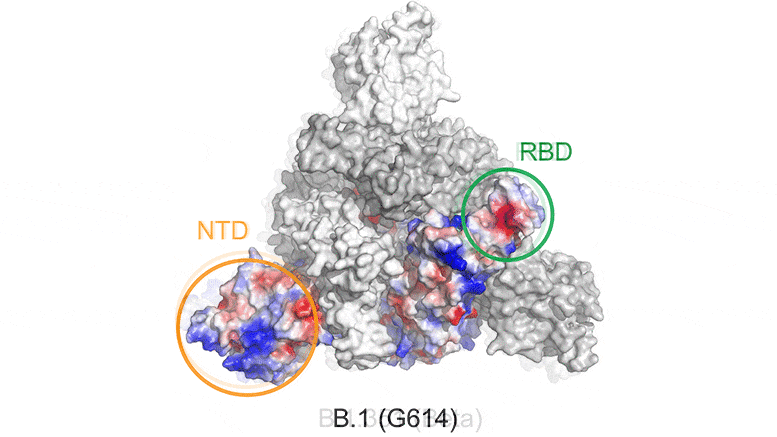
Mutations in the SARS-CoV-2 variants cause changes in the electrostatic potential (electrical charge at rest) on the spike surface. Here, positively charged areas are shown in blue and negatively charged areas in red. In the beta variant, the receptor binding domain (RBD) and N-terminal domain (NTD) are significantly altered, affecting the ability of antibodies to bind to and neutralize the virus. Credit: Bing Chen, PhD, Boston Children’s Hospital
Changes in the ‘spike’ protein explain the faster spread of Alpha and how the beta variant evades immune responses, suggesting a booster is needed with an updated vaccine.
New SARS-CoV-2 variants are spreading rapidly and it is feared that current COVID-19 vaccines will not protect against them. The latest in a series of structural studies of the “spike” protein of the SARS-CoV-2 variants, led by Bing Chen, PhD, at Boston Children’s Hospital, reveals novel properties of the Alpha (formerly UK) and Beta ( formerly South Africa) variants. Note that it suggests that current vaccines may be less effective against the beta variant.
Spike proteins, on the surface of SARS CoV-2, allow the virus to attach to and enter our cells, and all current vaccines target them. The new study, published in Science on June 24, 2021, used cryoelectron microscopy (cryo-EM) to compare the spike protein of the original virus with that of its Alpha and Beta variants.
The structural findings indicate that mutations in the Beta variant (also known as B.1.351) alter the shape of the spike surface at certain locations. As a result, neutralizing antibodies induced by current vaccines are less able to bind to the beta virus, potentially allowing it to escape the immune system even when people are vaccinated.
“The mutations make antibodies stimulated by the current vaccine less effective,” says Chen of Boston Children’s Department of Molecular Medicine. “The beta variant is somewhat resistant to current vaccines and we think a booster with the new genetic sequence could be beneficial for protection against this variant.”
However, the study also showed that mutations in the beta variant make the spike less effective at binding to ACE2 – suggesting that this variant is less transmissible than the alpha variant.
Reassurance about the Alpha variant; more variant studies underway
As for the Alpha variant (B.1.1.7), the study confirms that a genetic change in the spike (a single amino acid substitution) helps the virus bind better to ACE2 receptors, making it more infectious. However, tests indicate that antibodies raised by existing vaccines can still neutralize this variant.
To be a heightened threat, the researchers say, a SARS-CoV-2 variant would have to do three things: spread more easily, evade the immune system in vaccinated people or those previously exposed to COVID-19, and a more severe one. cause disease. Fortunately, the Alpha and Beta variants do not meet all these criteria.
“Our data suggest that the most problematic combination of such mutations is not yet present in the existing variants studied here,” the researchers write.
Chen’s team plans to also report the structures of other variants of concern, including the Delta variant (B.1.617.2) in the near future. Those investigations are still ongoing.
Reference: “Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants” by Yongfei Cai, Jun Zhang, Tianshu Xiao, Christy L. Lavine, Shaun Rawson, Hanqin Peng, Haisun Zhu, Krishna Anand, Pei Tong, Avneesh Gautam, Shen Lu, Sarah M. Sterling, Richard M. Walsh Jr., Sophia Rits-Volloch, Jianming Lu, Duane R. Wesemann, Wei Yang, Michael S. Seaman, and Bing Chen, June 24, 2021, Science.
DOI: 10.1126/science.abi9745
Yongfei Cai, PhD, Jun Zhang, PhD, and Tianshu Xiao, PhD of Boston Children’s Hospital were co-first authors on the paper. The study was funded by Emergent Ventures, the Massachusetts Consortium on Pathogen Readiness and the National Institutes of Health (grants AI147884, AI141002 and AI127193).










