Scientists Discover Opportunity To Disrupt SARS-CoV-2 Dynamics, Prevent COVID-19 Transmission
0 View
Share this Video
- Publish Date:
- 1 September, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
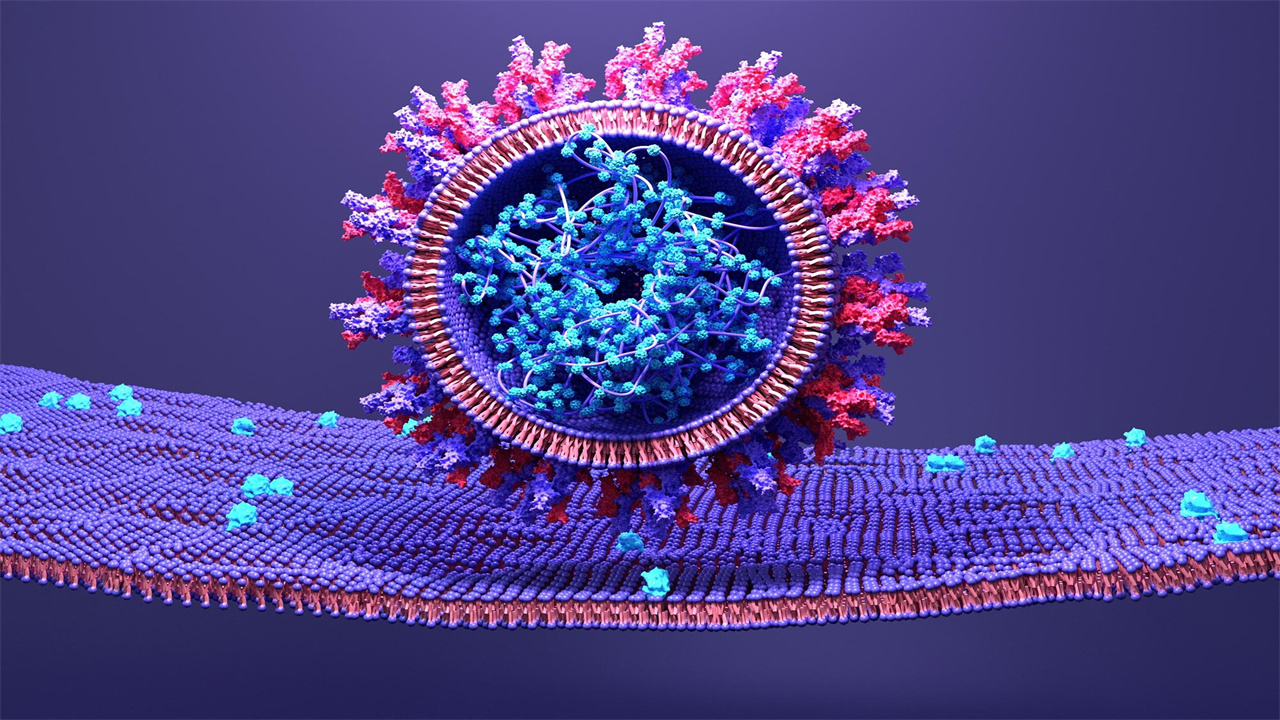
SARS-CoV-2 uses its spike protein to attach to a host cell.
A structural model of the SARS-CoV-2 spike protein as the virus fuses with human host cells reveals a possibility to disrupt dynamics and halt transmission.
Scientists have simulated the transition of the SARS-CoV-2 spike protein structure from the time it recognizes the host cell to the time it enters, according to a study published Aug. 31, 2021 in eLife.
The research shows that a structure enabled by sugar molecules on the spike protein may be essential for cell entry, and disrupting this structure could be a strategy to stop virus transmission.
An essential aspect of the SARS-CoV-2 life cycle is its ability to attach to host cells and transfer their genetic material. It achieves this through its spike protein, which consists of three separate components: a transmembrane bundle that anchors the spike to the virus, and two S subunits (S1 and S2) on the outside of the virus. To infect a human cell, the S1 subunit binds to a molecule on the surface of human cells called ACE2, and the S2 subunit detaches and fuses the viral and human cell membranes. Although this process is known, the exact order in which it occurs has not yet been discovered. But understanding the microsecond-scale and atomic-level movements of these protein structures could reveal potential targets for COVID-19 treatment.
“Most of current SARS-CoV-2 treatments and vaccines target the ACE2 recognition step of virus invasion, but an alternative strategy is to target the structural change that allows the virus to fuse with the human host cell,” explains study out. -author José N. Onuchic, Harry C & Olga K Wiess Professor of Physics at Rice University, Houston, USA, and co-director of the Center for Theoretical Biological Physics. “But experimentally examining these intermediate, temporal structures is extremely difficult, so we used a computer simulation that was simplified enough to examine this large system, but that preserves enough physical detail to capture the dynamics of the S2 subunit while it transitions between pre-fusion and post-fusion forms.”
The team was particularly interested in the role of sugar molecules on the spike protein, called glycans. To see whether the number, type and position of glycans play a role in the membrane fusion stage of viral cell entry by mediating these intermediate peak formations, they performed thousands of simulations using an all-atom model. Such models allow you to predict the trajectory of atoms over time, taking steric forces into account — that is, how neighboring atoms affect the motion of others.
The simulations revealed that glycans form a ‘cage’ that traps the ‘head’ of the S2 subunit, causing it to pause in an intermediate state between when it detaches from the S1 subunit and when the viral and cell membranes are fused. When the glycans were not there, the S2 subunit spent much less time in this conformation.
The simulations also suggest that holding the S2 head in a certain position helps the S2 subunit recruit and fuse human host cells with their membranes, by allowing the elongation of short proteins called fusion peptides from the virus. Indeed, glycosylation of S2 significantly increased the likelihood that a fusion peptide would spread to the host cell membrane, whereas when glycans were absent, there was only a marginal possibility that it would.
“Our simulations indicate that glycans can induce a pause during the peak protein transition. This presents a critical opportunity for the fusion peptides to capture the host cell,” concluded co-author Paul C. Whitford, associate professor at the Center for Theoretical Biological Physics. and Department of Physics, Northeastern University, Boston, USA “In the absence of glycans, the viral particle would probably not enter the host. Our study shows how sugars can control infectivity, and it provides a basis for experimental research into factors that influence the dynamics of this ubiquitous and deadly pathogen.”
Reference: “Sterically Restricted Rearrangements of SARS-CoV-2 Spike Protein Control Cell Invasion” by Esteban Dodero-Rojas, Jose N Onuchic, and Paul Charles Whitford, Aug. 31, 2021, eLife.
DOI: 10.7554 / eLife.70362










