Scientists Design “Nanotraps” to Catch and Clear Coronavirus From Tissue
0 View
Share this Video
- Publish Date:
- 10 May, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
A scanning electron microscope image of a nanotrap (orange) binding a simulated SARS-CoV-2 virus (dots in green). Scientists at the University of Chicago created these nanoparticles as a potential treatment for COVID-19. Credit: Courtesy of Chen and Rosenberg et al.
Potential COVID-19 treatment links nanoparticles to the immune system to search for and destroy viruses.
Researchers at the University of Chicago have designed a completely new potential treatment for COVID-19: nanoparticles that trap SARS-CoV-2 viruses in the body and then use the body’s own immune system to destroy them.
These “nanotraps” attract the virus by mimicking the target cells that the virus infects. When the virus binds to the nanotraps, the traps sequester the virus from other cells and target it for destruction by the immune system.
In theory, these nanotraps could also be used on variants of the virus, which could lead to a possible new way to inhibit the virus in the future. Although the therapy is still in the early stages of testing, the researchers envision it could be administered through a nasal spray as a treatment for COVID-19.
The results were recently published in the journal Matter.
“Since the start of the pandemic, our research team has developed this new way to treat COVID-19,” said Asst. Prof. Jun Huang of the Pritzker School of Molecular Engineering, whose lab led the research. “We’ve conducted rigorous testing to prove these nanotraps work, and we’re excited about their potential.”
Designing the perfect fall
To design the nanotrap, the research team – led by postdoctoral researcher Min Chen and graduate student Jill Rosenberg – examined the mechanism that SARS-CoV-2 uses to bind to cells: a spike-like protein on the surface that forms ACE2. receptor protein.
To create a trap that would similarly bind to the virus, they designed nanoparticles with a high density of ACE2 proteins on their surface. Likewise, they designed other nanoparticles with neutralizing antibodies on their surface. (These antibodies are produced in the body when someone is infected and are designed to hold onto the coronavirus in different ways).
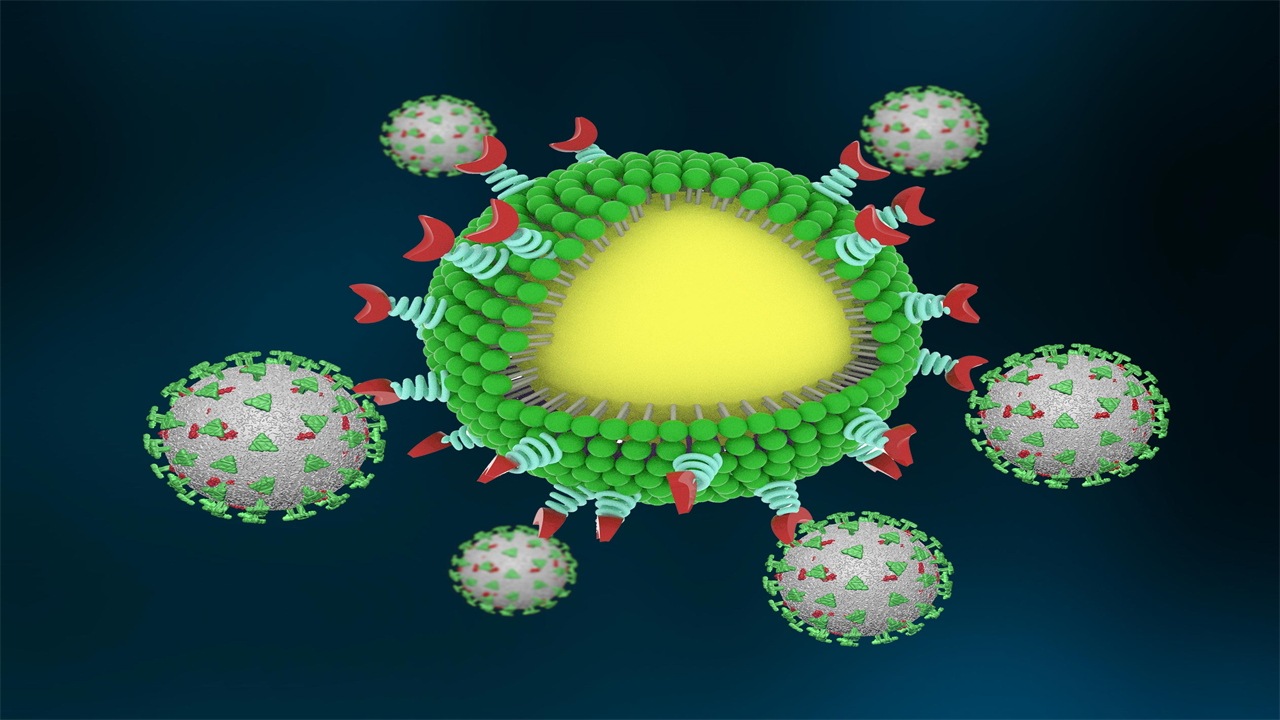
An artist’s concept of the nanotrap in action. The nanotrap is shown with a yellow core, a green phospholipid shell, and red functionalized particles to bind the virus (shown in gray, decorated with their infamous spike protein in green). Credit: Courtesy of Chen and Rosenberg et al.
The nanoparticles are made of FDA-approved polymers and phospholipids and are approximately 500 nanometers in diameter – much smaller than a cell. That means the nanotraps can reach more areas in the body and trap the virus more effectively.
To check that the tiny particles looked as they expected, they worked with Assoc’s lab. Prof. Bozhi Tian to use electron microscopes to get a good picture. “Based on our imaging, we saw a solid core and a lipid bilayer shell. That’s the essential part because it mimics the cell, ”says Tian, who is assigned to the Chemistry Department.
The researchers tested the safety of the system in a mouse model and found no toxicity. They then tested the nanotraps against a pseudovirus – a less potent model of a virus that does not replicate – in human lung cells in tissue culture plates and found that they completely blocked access to the cells.
Once the pseudovirus bound to the nanoparticle – which, in tests, took about 10 minutes after injection – the nanoparticles used a molecule that calls the body’s macrophages to gobble up and break down the nanoparticle. Macrophages generally eat nanoparticles in the body, but the nanotrap molecule speeds up the process. The nanoparticles were cleared and degraded within 48 hours.
The researchers also tested the nanoparticles with a pseudovirus in an ex vivo lung perfusion system – a pair of donated lungs kept alive with a ventilator – and found that they completely blocked infection in the lungs.
They also worked with researchers at Argonne National Laboratory to test the nanotraps with a live virus (rather than a pseudovirus) in an in vitro system. They found that their system inhibited the virus 10 times better than neutralizing antibodies or soluble ACE2 alone.
A possible future treatment for COVID-19 and beyond
The researchers then hope to test the system further, including more tests with a live virus and the many virus variants.
“That’s what’s so powerful about this nanotrap,” said Rosenberg. “It is easy to modulate. We can turn off different antibodies or proteins or target different immune cells depending on what we need with new variants. “
The nanotraps can be stored in a standard freezer and finally administered via an intranasal spray, placing them directly in the respiratory system and making them most effective.
The nanotraps can be stored in a standard freezer and could eventually be administered via an intranasal spray.
The researchers say it is also possible to act as a vaccine by optimizing the formulation.
“This nanomaterials approach provides a versatile platform to remove viruses and paves the way for the design of next-generation vaccines and therapies,” said study co-author and graduate student Jiuyun Shi.
“This is the starting point,” said Huang. “We want to do something to help the world.”
The study involved employees from various departments, including chemistry, biology and medicine. Other authors of the newspaper include Xiaolei Cai, Andy Chao Hsuan Lee, Jiuyun Shi, Mindy Nguyen, Thirushan Wignakumar, Vikranth Mirle, Arianna Joy Edobor, John Fung, Jessica Scott Donington, Kumaran Shanmugarajah, Yiliang Lin, Eugene Chang, Glenn Randall, Pablo Penaloza-MacMaster, Bozhi Tian and Maria Lucia Madariaga.
Reference: “Nanotraps for the Containment and Clearance of SARS-CoV-2” By Min Chen, Jillian Rosenberg, Xiaolei Cai, Andy Chao Hsuan Lee, Jiuyun Shi, Mindy Nguyen, Thirushan Wignakumar, Vikranth Mirle, Arianna Joy Edobor, John Fung, Jessica Scott Donington, Kumaran Shanmugarajah, Yiliang Lin, Eugene Chang, Glenn Randall, Pablo Penaloza-MacMaster, Bozhi Tian, Maria Lucia Madariaga and Jun Huang, 19 April 2021, Matter.
DOI: 10.1016 / j.matt.2021.04.005
Funding: National Institutes of Health, National Science Foundation, NIDDK.










