New Hope in the Battle Against COVID-19
0 View
Share this Video
- Publish Date:
- 27 August, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
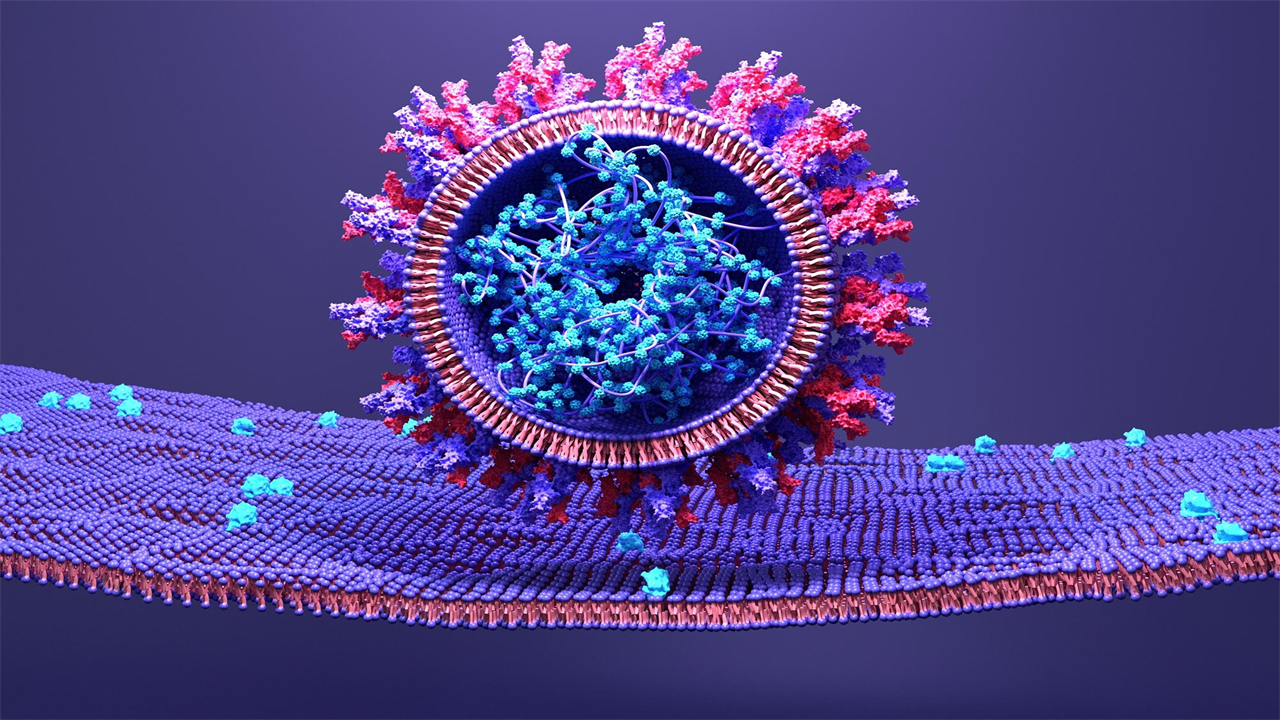
SARS-CoV-2 uses its spike protein to attach to a host cell.
Therapeutic approach developed by scientists at the Weizmann Institute could offer new hope in the fight against COVID-19.
While vaccines may send the world to a post-pandemic normal, a constantly mutating SARS-CoV-2 requires the development of effective drugs. In a new study published in Nature Microbiology, researchers at the Weizmann Institute of Science, along with collaborators from the Pasteur Institute, France, and the National Institutes of Health (NIH), USA, offer a new therapeutic approach to combat the infamous virus. Rather than targeting the viral protein responsible for the virus’s entry into the cell, the team of researchers focused on the protein on our cell’s membrane that allows this entry. Using an advanced artificial evolution method they developed, the researchers generated a molecular “super-cork” that physically blocks this “gateway”, preventing the virus from attaching to and entering the cell.
Prof. dr. Gideon Schreiber. A new approach to treating COVID-19 developed through artificial evolution. Credit: Weizmann Institute of Science
Most potential therapies (and current vaccines) for SARS-CoV-2 target the so-called “spike protein” found on the outer envelope of the virus. However, this protein is susceptible to mutations that affect the efficacy of these treatments. “Since the virus is constantly evolving, we instead focused on the non-evolving human receptor called ACE2 that acts as a gateway for the virus,” said Prof. Gideon Schreiber of Weizmann’s Department of Biomolecular Sciences, who oversaw the development of the virus. new study. This approach is not susceptible to new emerging virus variants, which is one of the main challenges in fighting the pandemic.
ACE2, attached to the membrane of lung epithelial cells and other tissues, is an enzyme important for regulating blood pressure. Therefore, tempting as it may be to simply block this receptor to prevent the entry of SARS-CoV-2, such a strategy should not interfere with the function of ACE2. Schreiber, whose lab specializes in studying interactions between proteins, wanted to develop a small protein molecule that could bind to ACE2 better than SARS-CoV-2, but without affecting the enzymatic activity of the receptor.
The researchers, led by Dr. Jiří Zahradník, a postdoctoral fellow in Schreiber’s group, set out to identify the binding domain of SARS-CoV-2: the relatively short string of building blocks in the larger spike protein that physically binds to ACE2. Using the virus’ own receptor-binding domain as a weapon against it, Zahradník performed several rounds of ‘evolution-in-a-test-tube’ developed in Schreiber’s lab on a genetically engineered strain of baker’s yeast. Because yeast can be easily manipulated, Zahradník was able to quickly scan millions of different mutations that had accumulated over the course of this artificial evolution, a process that mimics natural evolution at a much faster rate. Ultimately, the goal was to find a small molecule that would be significantly “stickier” than the original viral version.
The rapid evolutionary process resulted in a small protein fragment with a binding capacity 1000 times stronger than that of the original viral binding domain
During this scanning process, Schreiber’s team provided strong evidence for the hypothesis that SARS-CoV-2 becomes more contagious when mutations enhance adaptation to ACE2. The researchers found that already after the first round of selection, the lab-created variants with tighter binding capacities to ACE2 mimicked the mutations present in the binding domains of the most contagious SARS-CoV-2 strains that occurred through natural evolution. such as the British variant (Alpha), the South African variant (Beta) and the Brazilian variant (Gamma). Surprisingly, the now-widespread Indian (Delta) variant relies on another trick to be more contagious — by evading partial detection by the immune system.
Structure of an ACE2 receptor (left), the original binding molecule (top right), and the newly designed “supercork” (bottom right), imaged by cryogenic electron microscopy performed by Staff Scientists Dr. Nadav Elad of Weizmann’s Chemical Research Support Department and Dr. Orly Dym of the Life Sciences Core Facilities department. The black outline indicates the binding site of the “super cork” on the ACE2 receptor. Credit: Weizmann Institute of Science
Finally, Zahradník isolated a small protein fragment with a binding capacity 1000 times stronger than that of the original binding domain from which it evolved. Not only did this “super cork” fit ACE2 like a glove, it was also found by Maya Shemesh and Shir Marciano, PhD students in Schreiber’s lab, to preserve ACE2’s enzymatic activity — just as the researchers intended. In addition, due to the strong binding, very low concentrations of the newly developed molecule were required to achieve the desired blocking effect.
Looking for a “super cork” that would block the ACE2 receptor, the researchers examined about 1,000,000,000 yeast mutants.
To develop a possible method to deliver the molecule as a drug, Schreiber and his team collaborated with Prof. Yinon Rudich of Weizmann’s Department of Earth and Planetary Sciences. Together with Dr. Ira Marton and Dr. Chunlin Li, they created an aerosol-based spray with which the developed molecule could be inhaled to patients.
So far, the researchers at the NIH have tested the developed formulation in hamsters infected with SARS-CoV-2. Preliminary results indicate that this treatment significantly reduces disease symptoms, suggesting it could be a potential drug. More preclinical studies will take place at the NIH in the near future.
Reference: “Prediction of SARS-CoV-2 variants and antiviral drug design are enabled by RBD in vitro evolution” by Jiří Zahradník, Shir Marciano, Maya Shemesh, Eyal Zoler, Daniel Harari, Jeanne Chiaravalli, Björn Meyer, Yinon Rudich, Chunlin Li, Ira Marton, Orly Dym, Nadav Elad, Mark G. Lewis, Hanne Andersen, Matthew Gagne, Robert A. Seder, Daniel C. Douek, and Gideon Schreiber, Aug. 16, 2021, Nature Microbiology.
DOI: 10.1038/s41564-021-00954-4
Prof. Gideon Schreiber’s research is supported by the Ben B. and Joyce E. Eisenberg Foundation; the Rene and Tillie Molho Family Trust; Miel de Botton; and the Yotam project.