Kids Are Using Soft Drinks to Fake Positive COVID-19 Tests – Here’s the Science and How to Spot It
0 View
Share this Video
- Publish Date:
- 8 July, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
Kids will always find cunning ways to avoid school, and the latest trick is to fake a positive COVID-19 lateral flow (LFT) test using soft drinks. So how do fruit juices, cola and devious kids fool the tests and is there a way to tell a fake positive result from a real one? I’ve tried to find out.
At first I thought it was best to check the claims so I broke open bottles of Coke and Orange juice and then dropped a few drops directly onto LFTs. Sure enough, a few minutes later, two lines appeared on each test, ostensibly the presence of the virus that causes COVID-19.
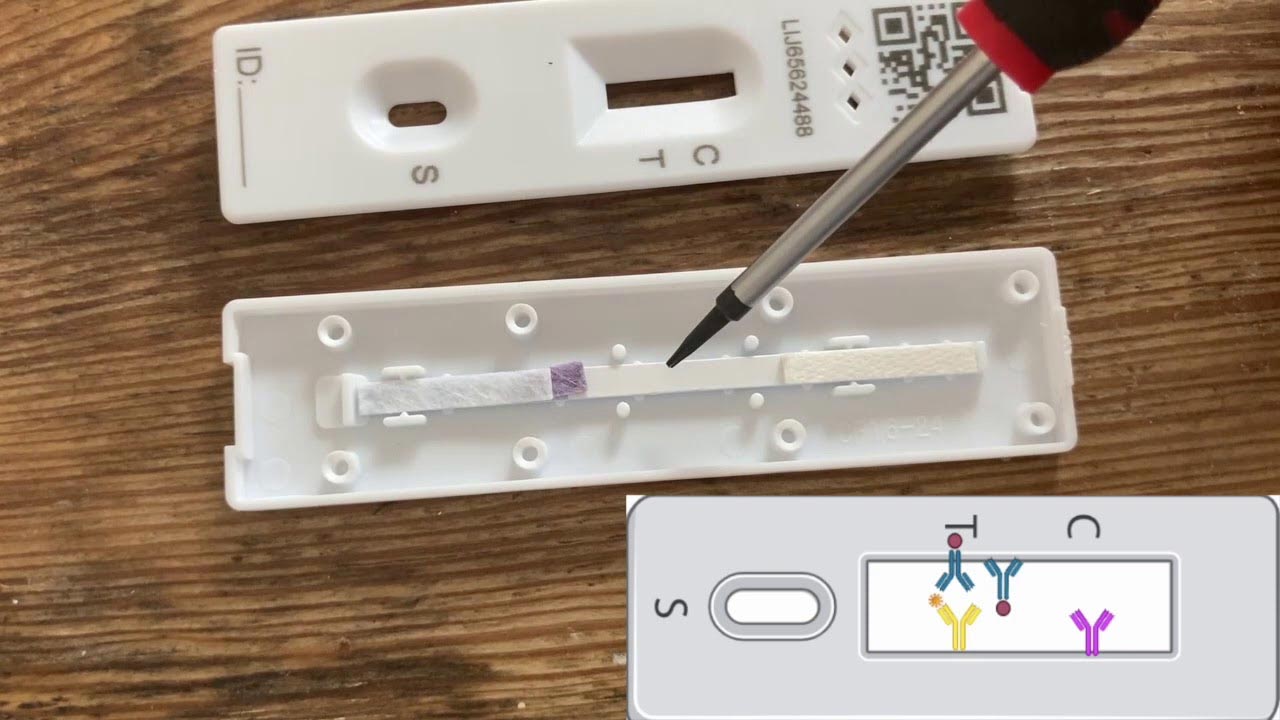
It’s worth understanding how the tests work. When you open an LFT device, you’ll find a strip of paper-like material called nitrocellulose and a small red pad hidden under the plastic casing below the T-line. The red pad absorbs antibodies that bind to the COVID-19 virus. They are also attached to gold nanoparticles (tiny gold particles actually appear red), allowing us to see where the antibodies are on the device. When doing a test, mix your sample with a liquid buffer solution to maintain the sample at an optimal pH before dropping it onto the strip.
False positive results. Credit: Mark Lorch
The liquid soaks up the nitrocellulose strip and takes up the gold and antibodies. The latter also bind to the virus, if present. Further down the strip, next to the T (for test), there are more antibodies that bind the virus. But these antibodies are not free to move – they are attached to the nitrocellulose. If the red smear of gold-labeled antibodies passes through this second set of antibodies, they also grab the virus. The virus then binds to both sets of antibodies — leaving everything, including the gold, immobilized on a line next to the T on the device, indicating a positive test.
Gold antibodies not bound to the virus continue on the strip where they meet a third set of antibodies, not designed to pick up COVID-19, which are stuck at the C line (for control). These capture the remaining gold particles, without having to do this via the virus. This last line is used to indicate that the test worked.
acid test
So how can a soda cause the appearance of a red T-line? One possibility is that the drinks contain something that the antibodies recognize and bind to, just like the virus. But this is quite unlikely. The reason antibodies are used in such tests is that they are incredibly picky about what they bind to. There’s all sorts of stuff in the snot and saliva collected by the swabs you take from the nose and mouth, and the antibodies completely ignore this mess of protein, other viruses, and leftovers from your breakfast. So they will not react to the ingredients of a soft drink.
A much more likely explanation is that something in the drinks is affecting the action of the antibodies. A range of liquids, from fruit juice to cola, have been used to mislead the tests, but they all have one thing in common: they are highly acidic. The citric acid in orange juice, phosphoric acid in cola, and malic acid in apple juice give these drinks a pH between 2.5 and 4. These are pretty harsh conditions for antibodies, which have evolved to work largely in the bloodstream, with its near neutral pH of about 7.4.
Maintaining an ideal pH for the antibodies is key to the proper function of the test, and that is the job of the liquid buffer solution you mix your sample with, which comes with the test. The critical role of the buffer is emphasized by the fact that if you mix Coke with the buffer – as evidenced by this debunking of an Austrian politician’s claim that mass testing is worthless – the LFTs behave exactly as you’d expect: negative for COVID -19.
So without the buffer, the antibodies in the test are fully exposed to the acidic pH of the drinks. And this has a dramatic effect on their structure and function. Antibodies are proteins, made up of amino acid building blocks, which are attached together to form long, linear chains. These chains fold into very specific structures. Even a small change in the chains can drastically affect the function of a protein. These structures are maintained by a network of many thousands of interactions between the different parts of the protein. For example, negatively charged parts of a protein are attracted to positively charged parts.
But in acidic conditions, the protein becomes increasingly positively charged. As a result, many of the interactions that hold the protein together are disrupted, the delicate structure of the protein is compromised and it no longer functions correctly. In this case, the sensitivity of the antibodies to the virus is lost.
Given this, you would expect the acidic drinks to result in completely blank tests. But denatured proteins are gooey beasts. All those perfectly evolved interactions that would normally hold the protein together are now orphaned and looking for something to bind to. So a likely explanation is that the immobilized antibodies at the T-line stick directly to the gold particles as they pass, producing the infamous Coke-induced false-positive result.
So is there a way to spot a false positive test? The antibodies (like most proteins) are able to refold and regain their function when returned to more favorable conditions. So I tried to wash a test that had been dripped with cola with buffer solution, and indeed, the immobilized antibodies at the T-line restored their normal function and released the gold particles, revealing the true negative result of the test.
Upstairs, LFT with Coke. Soil the same LFT later washed with buffer. Credit: Mark Lorch
Children, I applaud your ingenuity, but now that I have found a way to discover your deception, I suggest that you use your cunning to devise a series of experiments and test my hypothesis. Then we can publish your results in a peer-reviewed journal.
Written by Mark Lorch, Professor of Science Communication and Chemistry, University of Hull.
Originally published on The Conversation.










