Hidden SARS-CoV-2 “Gate” Discovered – Opens To Allow COVID Infection
0 View
Share this Video
- Publish Date:
- 27 August, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
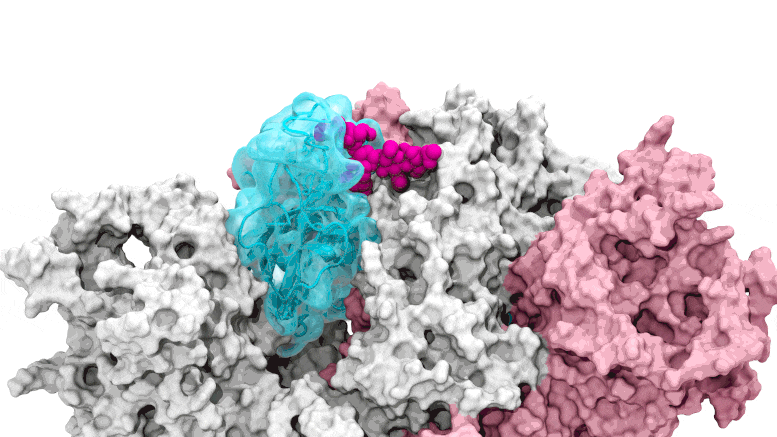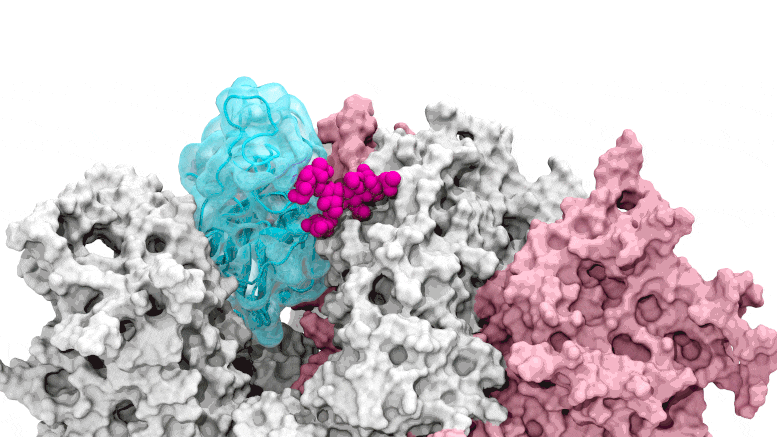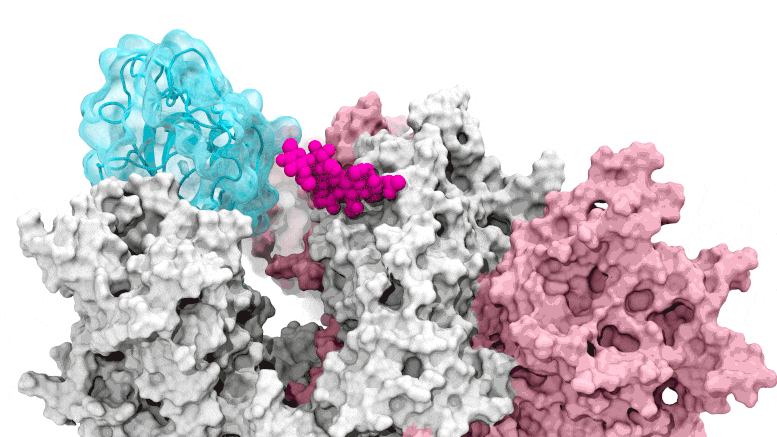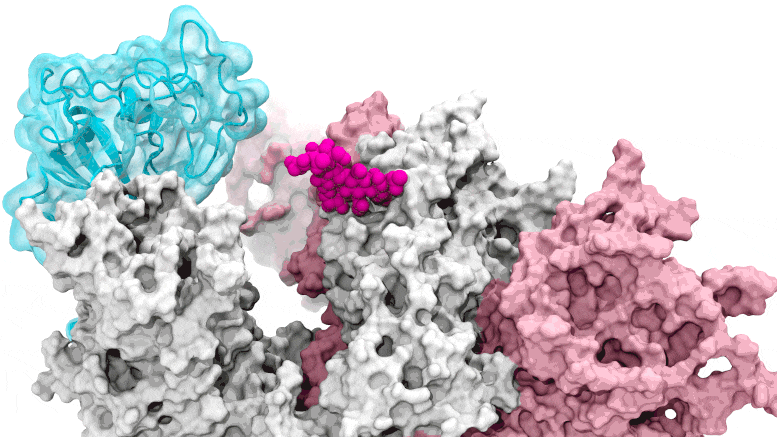
The glycan gate opens: Supercomputing-driven simulations show the glycan N343 (magenta) acting as a molecular crowbar to pry open the receptor-binding domain of the SARS-CoV-2 peak, or RBD (cyan), from a “down” to an “up” position. Credit: Terra Sztain, Surl-Hee Ahn, Lorenzo Casalino (Amaro Lab, UC San Diego)
Supercomputing-derived movies reveal details of a deceptive sugar coating on spike protein, offering new possibilities to block cell entry and infection.
Since the early days of the COVID pandemic, scientists have been aggressively pursuing the secrets of the mechanisms that allow severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to invade and infect healthy human cells.
Early in the pandemic, Rommie Amaro, a computational biophysical chemist at the University of California, San Diego, helped develop a detailed visualization of the SARS-CoV-2 spike protein that efficiently attaches to our cell receptors.
Now, Amaro and her research colleagues from UC San Diego, University of Pittsburgh, University of Texas at Austin, Columbia University and University of Wisconsin-Milwaukee have discovered how glycans — molecules that form a sugary residue around the edges of the spike protein — act as gateways for infections.
Published Aug. 19 in the journal Nature Chemistry, a study led by Amaro, co-senior author Lillian Chong at the University of Pittsburgh, first author and UC San Diego graduate student Terra Sztain and co-first author and UC San Diego postdoctoral researcher Surl -Hee Ahn, describes the discovery of glycan “gates” that open to let SARS-CoV-2 in.
“We essentially figured out how the spike actually opens and infects,” said Amaro, a professor of chemistry and biochemistry and a senior author on the new study. “We’ve unlocked an important secret of the spike in how it infects cells. Without this gate, the virus is basically incapable of being infected.”
Supercomputing-driven simulations show the glycan N343 (magenta) acting as a molecular crowbar to pry the receptor binding domain of the SARS-CoV-2 spike, or RBD (cyan), from a “down” to an “up” position . Credit: Terra Sztain, Surl-Hee Ahn, Lorenzo Casalino (Amaro Lab, UC San Diego)
Amaro believes the discovery of the gate by the research team opens potential avenues for new therapies to counter SARS-CoV-2 infection. If glycan gates could be pharmacologically locked in the closed position, the virus is effectively prevented from opening for entry and infection.
The coating of glycans on the spike helps trick the human immune system, as it comes across as nothing more than a sugary residue. Previous technologies that imaged these structures imaged glycans in static open or closed positions, which didn’t initially attract much interest from scientists. Supercomputing simulations then allowed the researchers to develop dynamic movies that revealed glycan gates activating from one position to another, providing an unprecedented slice of the infection story.
“We really got to see the opening and closing,” said Amaro. “That’s one of the really cool things these simulations give you: the ability to see really detailed movies. When you look at it, you realize that you are seeing something that we would have otherwise ignored. You just look at the closed structure, and then you look at the open structure, and it doesn’t look like anything special. Just because we filmed the whole process, you can really see it doing its thing.”
“Standard techniques would have taken years to simulate this opening process, but with the advanced simulation tools of my lab’s ‘weighted ensemble,’ we were able to capture the process in just 45 days,” Chong said.
The computationally intensive simulations were run first on Comet at the San Diego Supercomputer Center at UC San Diego and later on Longhorn at the Texas Advanced Computing Center at UT Austin. Such computing power offered the researchers an atomic-level view of the spike protein receptor binding domain, or RBD, from more than 300 perspectives. The studies revealed glycan “N343” as the pivot that pushes the RBD from the “down” to “up” position to access the host cell’s ACE2 receptor. The researchers describe N343 glycan activation as being similar to a “molecular crowbar” mechanism.
Jason McLellan, an associate professor of molecular biosciences at UT Austin, and his team created variants of the spike protein and tested how a lack of the glycan gate affected the RBD’s ability to open.
“We showed that without this gate, the spike protein RBD cannot adopt the conformation it needs to infect cells,” McLellan said.
Reference: “A glycan gate regulates the opening of the SARS-CoV-2 spike protein” by Terra Sztain, Surl-Hee Ahn, Anthony T. Bogetti, Lorenzo Casalino, Jory A. Goldsmith, Evan Seitz, Ryan S. McCool, Fiona L Kearns, Francisco Acosta-Reyes, Suvrajit Maji, Ghoncheh Mashayekhi, J. Andrew McCammon, Abbas Ourmazd, Joachim Frank, Jason S. McLellan, Lillian T. Chong, and Rommie E. Amaro, Aug. 19, 2021, Nature Chemistry.
DOI: 10.1038/s41557-021-00758-3
The full list of authors includes: Terra Sztain, Surl-Hee Ahn, Anthony Bogetti, Lorenzo Casalino, Jory Goldsmith, Evan Seitz, Ryan McCool, Fiona Kearns, Francisco Acosta-Ryes, Suvrajit Maji, Ghoncheh Mashayekhi, J. Andrew McCammon, Abbas Ourmazd, Joachim Frank, Jason McLellan, Lillian Chong and Rommie Amaro.










