Drugs Used to Treat COVID-19 Patients Tracked Throughout the Pandemic
0 View
Share this Video
- Publish Date:
- 21 May, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
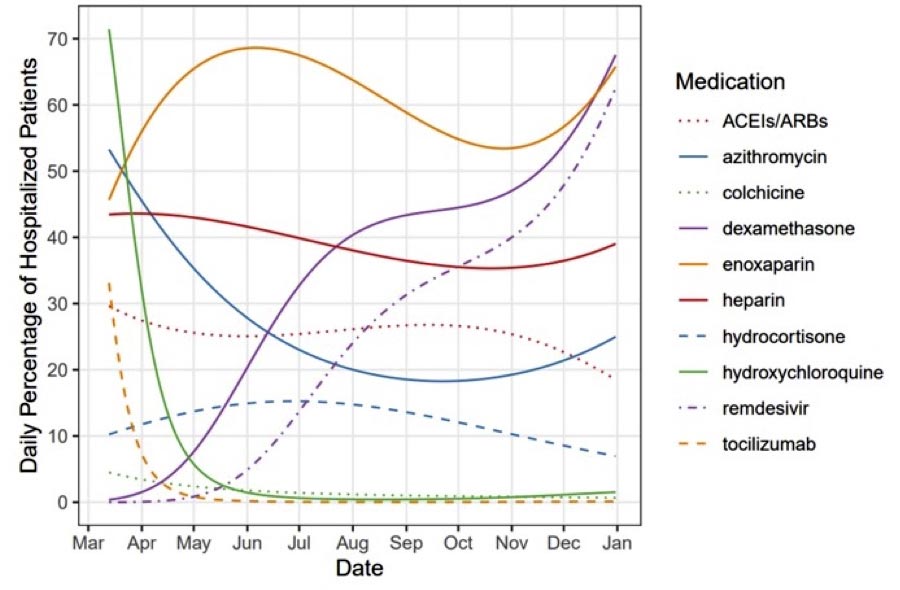
Patterns of COVID-19 drug use at UC Health medical facilities followed by UCI and UCSD Medical School investigators during the 2020 coronavirus pandemic. Credit: Jonathan Watanabe / UCI
Attempts to Treat COVID-19 Patients Described in UC Health Medications Data
A review of drug use patterns collected by an interdisciplinary team of researchers from the University of California, Irvine and the UC San Diego School of Medicine reveals the thinking, care, and scientific rigor that clinicians at UC Health’s medical centers apply to their treatment of patients with COVID -19 in 2020.
For a study published today (May 21, 2021) in the Journal of the American Medical Association Network Open, the researchers examined data on the use rates of 10 different drugs and drug categories to map out how drugs were used in people who were hospitalized. were included with the viral infection.
The authors obtained their data from the University of California’s COVID Research Data Set and followed 22,896 patients admitted to UC Health medical centers in Davis, Irvine, Los Angeles, San Diego and San Francisco between March 10 and December 31, 2020 .
“The home run of this paper is really in the numbers built from the UC CORDS database,” said lead author Jonathan Watanabe, UCI professor of clinical pharmacy. “You can clearly see how the use of certain drugs increased or decreased over the course of the pandemic and how those movements related to evidence-based decisions made in real time by UC health care providers. You can follow the evolution in the way we treat our sick patients. “
A strong example can be seen in the shift in acceptance of the antimicrobial drug hydroxychloroquine, which has been the subject of public discussions in White House briefings and substantial media coverage. In the early stages of the pandemic, the drug was given to more than 40 percent of patients, but use was less than 5 percent in June. Use of another drug in that class, azithromycin, fell from 40 to 30 percent over the same time frame.
“There were some studies conducted in the early part of the pandemic that were not particularly well designed and limited in size, showing that hydroxychloroquine was found to be useful,” said Watanabe, who is also the UCI’s co-founder for assessment and quality. from pharmacies. UCI’s School of Pharmacy & Pharmaceutical Sciences. “We saw high uptake of the drug early on, but then it just cratered because as time went on and more high-quality studies came in, it turned out to be ineffective.”
The opposite can be seen with dexamethasone, which increased from 1.4 percent of patients per day on March 31 to 67.5 percent at the end of December. According to Watanabe, the low-cost, generic corticosteroid has been shown to be effective in large studies involving hospital patients in the UK.
“On the face of it, many people might say that you wouldn’t want to use a corticosteroid that could, theoretically, reduce the immune response in a COVID patient,” he said. “But the studies really showed that the knee-shock mechanism of action-response was not correct in this case: the anti-inflammatory effect of the drug to tame cytokine storms was clearly more important than any weakening of the immune response.”
Remdesivir use grew 12-fold, from 4.9 percent on June 1 to 62.5 percent on December 31. Watanabe said one possible explanation for this is that the medication was only available in conjunction with studies in the UC system in the early part of the pandemic and became more widely distributed as time went on.
Enoxaparin, which is used to treat and prevent thrombosis, has also been shown to be effective against COVID-19, of which blood clots are a common symptom. The drug remained in use above 50 percent in 2020.
“We tend to give hospitalized patients an anticoagulant in general to reduce the risk of blood clots, which can happen because they are immobile in place for a long time,” Watanabe said. “But then we started to notice thrombophilia in COVID patients, so enoxaparin and heparin both became very important not only as prophylaxis but also as treatments.”
He noted that the usage figures in the paper show how physicians and other healthcare professionals responded effectively in real time to evidence and their own observations and that such information is important for clinicians to know for future planning purposes in terms of both treatment decision making as ensuring a robust delivery of proven drugs.
“This JAMA study is a thoughtful chronicle of the steps physicians, nurses and staff at UC Health’s medical centers took to help patients with potentially life-threatening illness,” said Jan Hirsch, founder and dean of UCI’s School of Pharmacy & Pharmacy. Pharmaceutical Sciences. “Not much was known at first about the correct course of treatment for COVID-19, but our people learned quickly and responded to evidence of what was effective on a daily and sometimes even more frequent basis.”
Reference: May 21, 2021, Journal of the American Medical Association Network Open.
Watanabe’s co-authors include Jimmy Kwon, a graduate student in the Statistics Department of UCI’s Donald Bren School of Information & Computer Sciences; Bin Nan, UCI professor of statistics; Shira Abeles, assistant clinical professor in the Division of Infectious Diseases & Global Public Health at UC San Diego School of Medicine; Stanley Jia, a UCI undergraduate research assistant in the Clinical Pharmacy Practice Department; and Sanjay Mehta, associate clinical professor in the UC San Diego School of Medicine’s Division of Infectious Diseases & Global Public Health.










