Deconstructing the Infectious Biological Weaponry of the COVID-19 Virus
0 View
Share this Video
- Publish Date:
- 8 August, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
This color-enhanced image, taken with scanning electron microscopy, shows massive amounts of SARS-CoV-2 particles (purple) bursting from kidney cells (green), which the virus has hijacked for replication. The bulging, spherical cells in the top right and bottom left corners are deformed and about to burst from the viral particles inside, starting to self-destruct. Credit: NIAID Integrated Research Facility
Scientists are working together to model the complex protein responsible for SARS-CoV-2 replication, revealing the potential vulnerabilities for drug development.
In February 2020, a trio of bioimaging experts were sitting comfortably around a dinner table at a science conference in Washington, DC, when the conversation shifted to what was then a worrying viral epidemic in China. Without foreseeing the global disaster to come, they wondered aloud how they could contribute.
Nearly a year and a half later, those three scientists and their many collaborators in three national labs published a comprehensive study in the Biophysical Journal that — among other recent, complementary studies of coronavirus proteins and genetics — represents the first step toward developing treatments for viral infections. infection, now seared into global consciousness as COVID-19.
Their fundamental work focused on the protein-based machinery that allows the SARS-CoV-2 virus to hijack the molecular machinery of our own cells to replicate in our bodies.
From structure to function to solutions
“It has been noted that all organisms are just a means for DNA to make copies of itself, and nowhere is this more true than in the case of a virus,” said Greg Hura, a staff scientist at Lawrence Berkeley National Laboratory (Berkeley Lab). ) and one of the study’s lead authors. “A virus’s unique job is to make copies of its genetic material — unfortunately at our expense.”
Viruses and mammals, including humans, have been locked in this battle for millions of years, he added, during which time the viruses have developed many tricks to get their genes copied into us, while our bodies have developed counter-defences. And while viruses often perform a long list of other activities, their ability to harm us with infection really comes down to whether they can replicate their genetic material (RNA or DNA, depending on the species) to make more viral particles and use our cells to translate their genetic code into proteins.
The protein-based machinery responsible for RNA replication and translation in coronaviruses — and many other viruses — is called the RNA transcription complex (RTC), and it is a truly formidable piece of biological weaponry.
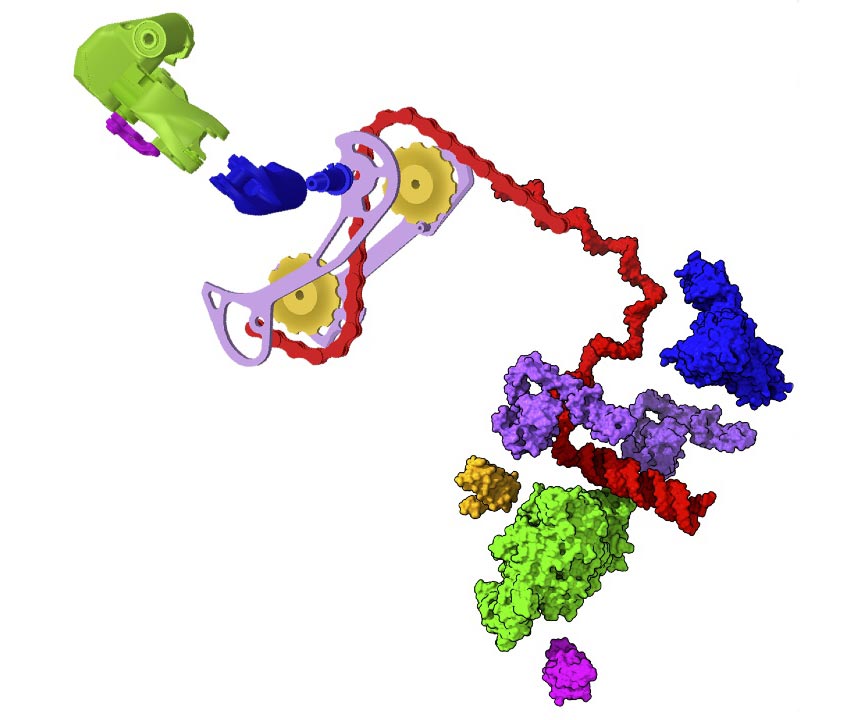
A view of the SARS-CoV-2 machine illustrating its ability to quickly change structural arrangements — such as a bicycle changing gears — to perform various tasks. Credit: Greg Hura/Berkeley Lab
To successfully duplicate viral RNA for new virus particles and produce the many proteins of the new particles, the RTC must: distinguish between viral and host RNA, recognize and link RNA bases instead of very similar DNA bases that also abundant in human cells, their RNA into mRNA (to duplicate human ribosomes in translating viral proteins), interface with molecules that control errors, and transcribe specific sections of viral RNA to amplify certain proteins over others, depending on need – while trying at all times to evade the host’s immune system that will recognize it as a foreign protein.
As amazing as this may sound, any newly developed virus that is successful “must have machines that are incredibly sophisticated to overcome the mechanisms we developed,” explains Hura, head of the Structural Biology Division in the Molecular Biophysics and Integrated Bioimaging Division of Berkeley Lab.
He and the other study leaders — Andrzej Joachimiak of Argonne National Laboratory and Hugh M. O’Neill of Oak Ridge National Laboratory — specialize in revealing the atomic structure of proteins to understand how they work at the molecular level. So the trio knew from the moment they first discussed COVID-19 at the dinner table that studying the RTC would be particularly challenging because multitasking protein machines like the RTC are not static or rigid, like molecular diagrams or ball-and-chest. -stick models might suggest. They are flexible and have associated molecules called non-structural and accessory proteins (Nsps) that exist in a myriad of rapidly rearranging forms depending on the task at hand — similar to how a gear lever on a bicycle quickly adjusts the vehicle to changing terrain.
Each of these Nsp arrangements provides insight into the different activities of the protein, and they also expose different parts of the total RTC surface, which can be examined to find places where potential drug molecules can bind and inhibit the whole machine.
So, after their accidental convergence in Washington, the trio devised a plan to pool their knowledge and national lab resources to document the structure of as many RTC arrangements as possible and identify how these shapes interact with other viral and human molecules.
Science during shutdowns
The research revolved around combining data collected from many advanced imaging techniques, as no single approach on its own can generate complete atomic-level blueprints of infectious proteins in their natural state. They combined small-angle X-ray scattering (SAXS), X-ray crystallography and small-angle neutron scattering (SANS) performed at Berkeley Lab’s Advanced Light Source, Argonne’s Advanced Photon Source and Oak Ridge’s High Flux Isotope Reactor and Spallation Neutron Source, respectively, on samples of biosynthetically produced RTC.
“Apart from the complexity of the viral system, working during the pandemic was very hard. But we were driven to conduct this research more than anything we’ve ever done by all the suffering families across the country and even the world have endured.” – Greg Hura, photographed working at the ALS beamline used for SAXS, in June 2020. Credit: Thor Swift/Berkeley Lab
Despite the extraordinary hurdles in conducting science during shelter-in-place conditions, the collaboration was able to run continuously for more than 15 months, thanks to research funding and facility operations support from the Department of Energy’s Office of Science National Virtual Biotechnology Laboratory ( NVBL). During that time, the scientists collected detailed data on the RTC’s major accessory proteins and their interactions with RNA. All of their findings were uploaded to the open-access Protein Data Bank prior to the publication of the journal article.
Among the many structural findings that will aid drug design, a remarkable discovery is that the assembly of the RTC subunits is incredibly accurate. Again using a mechanical metaphor, the scientists liken the assembly process to assembling a spring-based machine. You can’t put a spring when the rest of the machine is already in place, you have to compress the spring and put it on a certain mounting step or the whole device will stop working. Likewise, the RTC Nsps cannot come into place in any random or chaotic order; they must follow a specific sequence of operations.
They also identified how one of the Nsps specifically recognizes the RNA molecules it acts on, and how it cuts long strands of copied RNA into the correct length.
“Having the vaccines is definitely huge. But why are we content with just this one defense option?” said Hooray. Joachimiak added: “This was a survey study, and it identified many directions that we and others should follow very deeply; to tackle this virus, we need multiple ways to block its spread.”
“Combining information from different structural techniques and calculations will be key to achieving this goal,” O’Neill said.
Because of the similarity of RTC proteins between viral strains, the team believes that all drugs designed to block RTC activity, in addition to all COVID-19 variants, can also work for multiple viral infections.
Looking back at the beginning of their research journey, the scientists marvel at the fortunate timing of it all. When we started talking, Hura said, “we had no idea that this epidemic would soon become a generation-changing pandemic.”
Reference: “Transient and Stabilized Complexes of Nsp7, Nsp8, and Nsp12 in SARS-CoV-2 Replication” by Mateusz Wilamowski, Michal Hammel, Wellington Leite, Qiu Zhang, Youngchang Kim, Kevin L. Weiss, Robert Jedrzejczak, Daniel J. Rosenberg , Yichong Fan, Jacek Wower, Jan C. Bierma, Altaf H. Sarker, Susan E. Tsutakawa, and Sai Venkatesh, June 28, 2021, Biophysical Journal.
DOI: 10.1016/j.bpj.2021.06.006
This study was supported by the DOE Office of Science through NVBL, a consortium of national DOE laboratories focused on the response to COVID-19, with funding from the Coronavirus CARES Act; and by the National Institutes of Health. The Advanced Light Source, Advanced Photon Source, High Flux Isotope Reactor and Spallation Neutron Source are DOE Office of Science user facilities.










