Coronavirus’ Spike Protein Attacks Vascular System on a Cellular Level
0 View
Share this Video
- Publish Date:
- 2 May, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
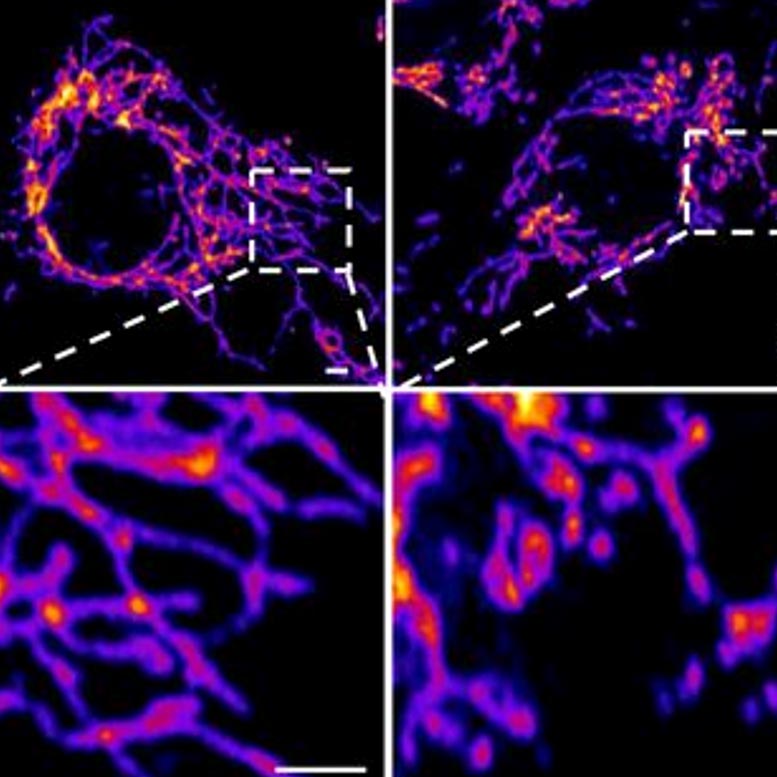
Representative images of vascular endothelial control cells (left) and cells treated with the SARS-CoV-2 Spike protein (right) show that the spike protein causes increased mitochondrial fragmentation in vascular cells. Credit: Salk Institute
Researchers and collaborators at Salk show how the protein damages cells, confirming that COVID-19 is primarily a vascular disease.
Scientists have known for some time that SARS-CoV-2’s signature ‘spike’ proteins help the virus infect its host by adhering to healthy cells. Now, a large new study shows that they also play a key role in the disease itself.
The paper, published April 30, 2021, in Circulation Research, also conclusively shows that COVID-19 is a vascular disease, showing exactly how the SARS-CoV-2 virus damages and attacks the vascular system at the cellular level. The findings help explain the wide variety of seemingly unrelated complications of COVID-19 and could open the door for new research into more effective therapies.
“Many people consider it a respiratory disease, but it is in fact a vascular disease,” said assistant professor Uri Manor, co-senior author of the study. That could explain why some people have strokes and why some people have problems with other parts of the body. The similarity between them is that they all have vascular underpinnings. “
Salk researchers collaborated with scientists at the University of California San Diego on the paper, including co-first author Jiao Zhang and co-senior author John Shyy, among others.
While the findings themselves are not entirely surprising, the paper provides clear confirmation and detailed explanation of the mechanism by which the protein first damages vascular cells. There is a growing consensus that SARS-CoV-2 affects the vascular system, but exactly how it did so has not been understood. Likewise, scientists studying other coronaviruses have long suspected that the spike protein contributed to damaging vascular endothelial cells, but this is the first time the process has been documented.
In the new study, the researchers created a “pseudovirus” that was surrounded by SARS-CoV-2 classic crown of spike proteins, but did not contain a real virus. Exposure to this pseudovirus resulted in damage to the lungs and arteries of an animal model, proving that the spike protein alone was sufficient to cause disease. Tissue samples showed inflammation in endothelial cells lining the pulmonary artery walls.
The team then replicated this process in the lab, exposing healthy endothelial cells (lining arteries) to the spike protein. They showed that the spike protein damaged cells by binding ACE2. This binding disrupted ACE2’s molecular signaling to mitochondria (organelles that generate energy for cells), causing damage and fragmentation of mitochondria.
Previous studies have shown a similar effect when cells were exposed to the SARS-CoV-2 virus, but this is the first study to show that the damage occurs when cells are exposed to the spike protein alone.
“If you remove the virus’s replication capability, it still has a major detrimental effect on the vascular cells simply because of its ability to bind to this ACE2 receptor, the S protein receptor, now famous for COVID,” explains Manor. . “Further studies with mutant spike proteins will also provide new insights into the infectivity and severity of mutant SARS CoV-2 viruses.”
Next, the researchers hope to take a closer look at the mechanism by which the disrupted ACE2 protein damages mitochondria and changes shape.
Reference: “SARS-CoV-2 Spike Protein Impairs Endothelial Function Via Downregulation of ACE 2” by Yuyang Lei, Jiao Zhang, Cara R. Schiavon, Ming He, Lili Chen, Hui Shen, Yichi Zhang, Qian Yin, Yoshitake Cho, Leonardo Andrade, Gerald S. Shadel, Mark Hepokoski, Ting Lei, Hongliang Wang, Jin Zhang, Jason X.-J. Yuan, Atul Malhotra, Uri Manor, Shengpeng Wang, Zu-Yi Yuan, and John YJ. Shyy, March 31, 2021, Circulation research.
DOI: 10.1161 / CIRCRESAHA.121.318902
Other authors of the study are Yuyang Lei and Zu-Yi Yuan from Jiaotong University in Xi’an, China; Cara R. Schiavon, Leonardo Andrade and Gerald S. Shadel of Salk; Ming He, Hui Shen, Yichi Zhang, Yoshitake Cho, Mark Hepokoski, Jason X.-J. Yuan, Atul Malhotra, Jin Zhang from the University of California San Diego; Lili Chen, Qian Yin, Ting Lei, Hongliang Wang, and Shengpeng Wang from Xi’an Jiatong University Health Science Center in Xi’an, China.
The research was supported by the National Institutes of Health, National Natural Science Foundation of China, Shaanxi Natural Science Fund, National Key Research and Development Program, Xi’an Jiaotong University’s First Affiliated Hospital; and Xi’an Jiaotong University.










