Clinical Trials for COVID-19 Drugs Have Had Inconsistent Results – Now Scientists Think They Know Why
0 View
Share this Video
- Publish Date:
- 6 July, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
Schematic illustration of the sample size calculation process. Credit: Shoya Iwanami
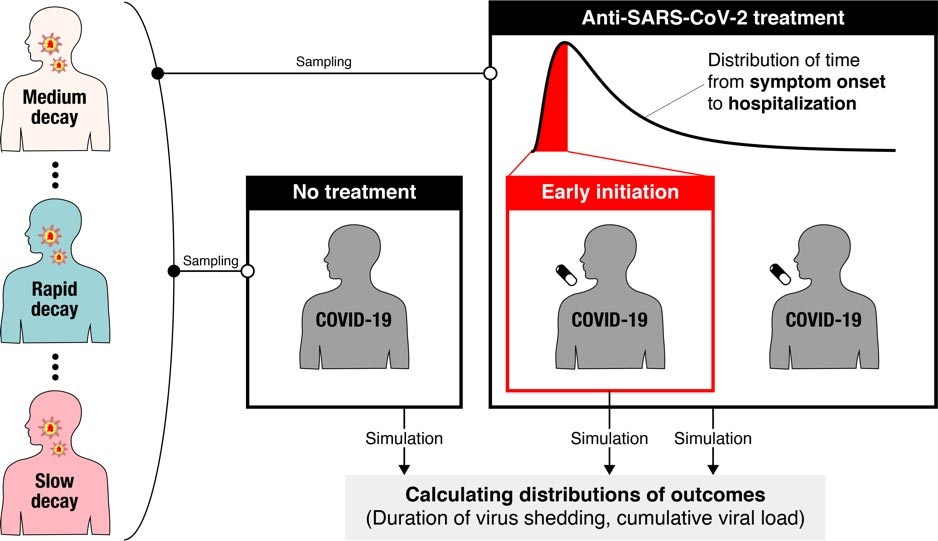
A new modeling study suggests that significant variation in virus dynamics from person to person may be a contributing factor to inconsistent findings reported in clinical trials of COVID-19 antiviral drugs. Recruiting trial participants soon after symptoms begin could reduce the number of participants needed to detect significant antiviral drug effects, according to Shoya Iwanami and Shingo Iwami of Nagoya University in Aichi, Japan, Keisuke Ejima of Indiana University, Indiana, US, and colleagues, who present these findings in the open-access journal PLOS Medicine.
An effective antiviral drug for COVID-19 would have significant global health benefits. However, clinical trials testing drug candidates have shown inconsistent results, perhaps due to flaws in the way the trials are designed.
To address this problem, Iwanami and colleagues first used a model of the dynamics of SARS-CoV-2 – the virus that causes COVID-19 – once it has infected a person. They combined the model with clinical data to examine how viral load (the amount of virus in a person’s throat) changes over time, and found significant variation in the rate of decline between patients. These differences may contribute to the inconsistent results reported in non-randomized clinical trials to date.
Exploring further, the researchers simulated possible findings from randomized clinical trials of COVID-19 drugs that successfully interrupt virus replication. They found that even if a drug reduced viral replication by 95%, the associated randomized clinical trial would have to enroll more than 13,000 people to receive the drug tested, plus the same number of people to receive a placebo for comparison, to detect statistically significant differences in viral load. In most cases, those numbers would be unreasonably large.
However, when the researchers changed the simulated randomized clinical trials so that participants were treated within one day of the onset of their symptoms, they found that only about 600 participants were needed for each group. This suggests that randomized clinical trials of COVID-19 drugs could be improved by enrolling participants as soon as possible after the onset of symptoms, or by establishing enrollment criteria based on the time elapsed since the onset of symptoms.
The researchers note that future studies could use more detailed models of SARS-CoV-2 dynamics to make more reliable calculations of the number of participants needed for randomized clinical trials to produce consistent results.
“We found that if patients are recruited into clinical trials, regardless of the time since the onset of symptoms, the number should be over 10,000, which is unreasonably high,” adds Dr. Iwami. “This is because many patients are recruited too late to see the effect of the antiviral treatment. Therefore, we recommend recruiting only those who are still ‘new’ since the onset of symptoms. If we only recruit patients within 2 days since the onset of symptoms, only 500 patients need to be recruited. The approach we developed is applicable to other types of drugs and other infectious diseases. We hope to develop an online platform that supports the design of clinical trials.”
Reference: “Detection of significant antiviral drug effects on COVID-19 with reasonable sample size in randomized controlled trials: a pilot study combined with clinical data” by Iwanami S, Ejima K, Kim KS, Noshita K, Fujita Y, Miyazaki T, et al., July 6, 2021, PLOS Medicine.
DOI: 10.1371/journal.pmed.1003660
Funding: This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP19J12319 (to S.Iwanami), JP18KT0018 (to SI), JP18H01139 (to SI), JP16H04845 (to SI), JP17H04085 (to KW ), JP18K18146 (to KE), JP20H05042 (to SI), JP19H04839 (to SI), JP18H05103 (to SI); Japan Agency for Medical Research and Development (AMED) Grant Numbers JP19gm1310002 (to SI), JP20wm0325007h0001 (to SI), JP20wm0325004s0201 (to SI), JP20wm0325012s0301 (to SI), JP20wm0325015s0301 (to SI25007), JP0hm0 (to SI), JP0hm0 SI), to SI), JP19fk0108156h0001 (to SI), JP20fk0108140s0801 (to SI), JP20fk0108413s0301 (to SI), JP19fk0210036h0502 (to SI), JP19fk0210036j0002 (to KW), JP19fk03 (000fk1014h0136), JP19fk0310114h01 (to SI), JP19fk0310103j0203 (to KW); Japan Science and Technology Agency (JST) MIRAI (vs SI and KW); Moonshot R&D Grant Number JPMJMS2021 (to KA and SI) and JPMJMS2025 (to SI); Mitsui Life Social Welfare Foundation (to SI and KW); Shin-Nihon of Advanced Medical Research (to SI); Suzuken Memorial Foundation (to SI); Life Science Foundation of Japan (to SI); SECOM Foundation for Science and Technology (to SI); Japan Prize Foundation (to SI); Daiwa Securities Health Foundation (vs SI); The Yasuda Medical Foundation (to KW); Smoking Research Foundation (after KW); and The Takeda Science Foundation (to KW); NSF PHY-2031756 (to ASP); NIH R01-OD011095 and R01-AI028433 (to ASP); and Los Alamos National Laboratory LDRD Program (to ASP); the MIDAS Coordination Center (MIDASSUGP2020-6) by a grant from the NIGMS (3U24GM132013-02S2) (to KE); Meiji Yasuda Life Foundation of Health and Welfare (KE). The funders had no role in the study design, data collection and analysis, the decision to publish the manuscript, or preparation of the manuscript.










