Blood Platelets Key to Deadly Organ Damage in COVID-19 Patients
0 View
Share this Video
- Publish Date:
- 9 September, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
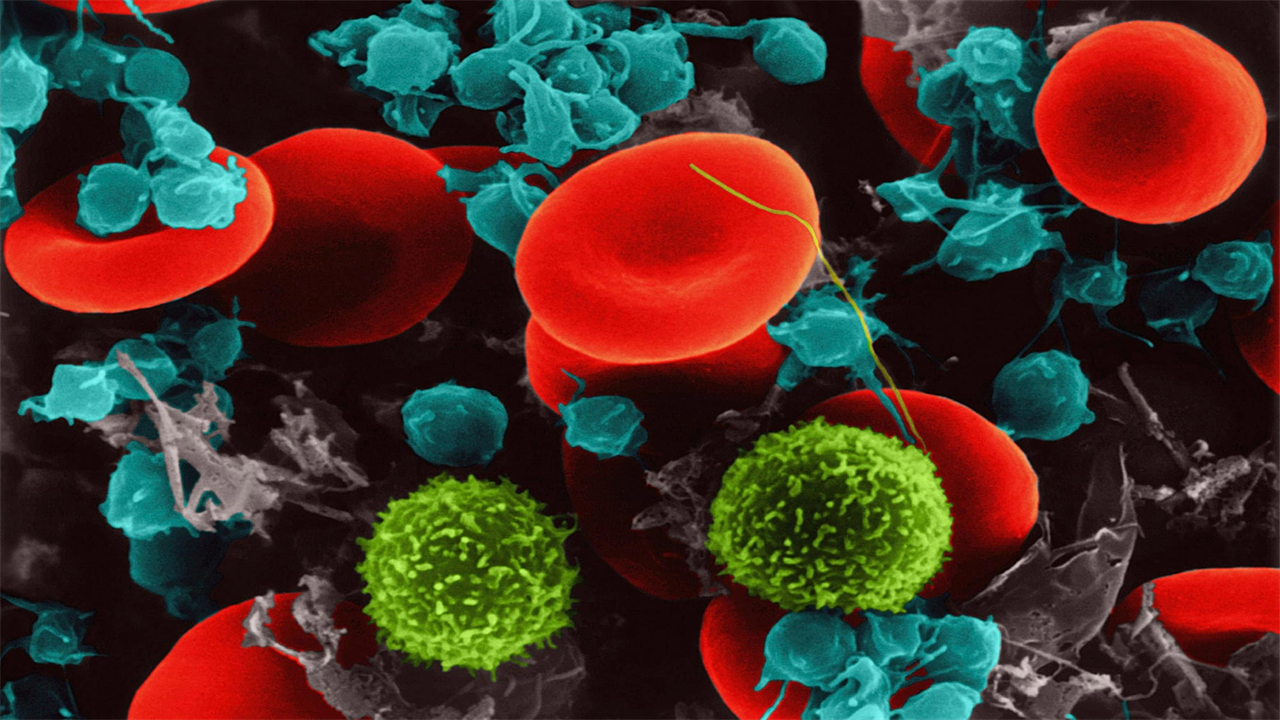
Abnormal crosstalk between platelets and cells that line blood vessels is a cause of deadly organ damage in patients with severe COVID-19, a new study finds.
Led by researchers at the NYU Grossman School of Medicine, the study revealed the protein signals released by platelets, cell fragments that contribute to blood clotting, cause inflammation, abnormal clotting and damage to blood vessels when exposed to the pandemic virus.
Published online Sept. 8 in Science Advances, the work identified two related genes, S1000A8 and S1000A9, that are found in the platelets of COVID-19 patients, causing them to make more of myeloid-related proteins (MRP) 8 and 14. Higher levels of the two proteins, known to work in pairs and present in high amounts in immune cells, were linked in the study to higher levels of clotting and inflammation in blood vessels, greater disease severity and longer hospital stays.
In support of the theory that platelets are at the core of blood vessel damage in COVID-19, the research team also presented evidence that approved drugs known to block platelet activation via the platelet surface protein P2Y12 (clopidogrel or ticagrelor) COVID-19. related inflammation in blood vessels. The study also found that platelets exposed to COVID-19 largely alter the cells that line blood vessels (endothelial cells) through a protein called p-selectin, which makes platelets stickier and more likely to form clots.
“Our findings reveal a new role for platelets in blood vessel damage from COVID-19, and may explain in large part what makes the COVID-19 virus so much more deadly than its cold-causing relatives,” said corresponding author Tessa. Barrett, PhD, research assistant professor in the Department of Medicine at NYU Langone Health.
Better understanding
Abnormal, body-wide inflammation and blood clotting were identified early in the pandemic as central features of severe COVID-19, with the two believed to be related, the study authors say. As blood components that respond to injuries in blood vessels by causing inflammation and becoming sticky to clump together in clots, platelets have been suggested as a culprit for the observed damage. Furthermore, there is mounting evidence that the interplay between platelets and endothelial cells may be important for these disease mechanisms.
For the current study, small blood vessel endothelial cells were exposed to fluid released from the platelets of either COVID-19 patients or healthy, comparable patients (controls). The genetic material (RNA) was then sequenced to read the order of the molecular “letters” that make up codes of active genes (transcriptions) in each case. In the presence of COVID-19-activated platelets, changes were observed in the activity of the endothelial cells exposed to them, with 485 transcripts becoming less active and 803 popping up. Genes otherwise expressed in COVID-19 were linked to clotting, inflammation and the weakening of connections between endothelial cells, which allows blood serum to seep into tissue to cause the pulmonary edema seen in severe cases, where patients’ lungs become dilated. fill with liquid.
From the initial large list of potential culprits, cross-referencing with databases reduced the candidate list to two related fragments of genetic material: S100A8 and S100A9, which coded for the build-up of MRP 8 and 14. The presence of COVID-19 in patients was found to increasing the amount of MRP8/14 produced by platelets and other cells by 166 percent compared to patients without the infection. Higher levels of MRP8/14 have been associated with abnormal clotting (thrombosis), inflammation and critical illness in hospitalized patients with COVID-19. Intriguingly, upregulation of S100A8/A9 did not occur after platelet exposure to a relative of the pandemic virus, CoV-OC43, that causes the common cold.
In addition, the research team found that platelet-driven endothelial damage and abnormal clotting can occur through the action of p-selectin in platelet components called alpha granules. Normally, p-selectin resides in the granules and ‘clips’ out when platelets are activated, where it promotes platelet clumping and signals that stimulate the local immune response.
The researchers also found that the anticoagulant P2Y12 inhibitors reduced the expression of S100A8 and S100A9 in platelets by 18 percent over four weeks, and in lab tests prevented COVID-19 platelets from causing damage to blood vessels.
“The current study supports the theory that platelets activate endothelial cells via P-selectin, and that both p-selectin and MRP8/14 contribute to vascular damage and an increased risk of death,” said senior study author Jeffrey S. Berger, MD, director of the Center for the Prevention of Cardiovascular Disease at NYU Grossman School of Medicine, and professor of medicine and surgery. “As our team also leads ACTIV4a, a large ongoing NIH-funded anticoagulant study in COVID-19, we are currently testing patients to see whether P2Y12 inhibitors can better prevent serious disease. Annual meeting of the association in November.”
ACTIV-4a will also soon begin testing the effect of a P-selectin inhibitor called crizanlizumab in patients hospitalized with COVID-19, Berger says. Targeting P-selectin can block both platelet and endothelial cell activation and their interactions – with P2Y12 inhibitors targeting only platelets.
Reference: “Platelets Enhance Endotheliopathy in COVID-19” by Tessa J. Barrett, MacIntosh Cornwell, Khrystyna Myndzar, Christina C. Rolling, Yuhe Xia, Kamelia Drenkova, Antoine Biebuyck, Alexander T. Fields, Michael Tawil, Elliot Luttrell-Williams, Eugene Yuriditsky, Grace Smith, Paolo Cotzia, Matthew D. Neal, Lucy Z. Kornblith, Stefania Pittaluga, Amy V. Rapkiewicz, Hannah M. Burgess, Ian Mohr, Kenneth A. Stapleford, Deepak Voora, Kelly Ruggles, Judith Hochman and Jeffrey S. Berger, September 8, 2021, Advances in Science.
DOI: 10.1126/sciaadv.abh2434
Along with Barrett and Berger, the authors of the NYU Langone Health study were MacIntosh Cornwell, Khrystyna Myndzar, Christina Rolling, Yuhe Xia, Kamelia Drenkova, Antoine Biebuyck, Michael Tawil, Elliot Luttrell-Williams, Eugene Yuriditsky, and Judith Hochman from the Department of Medicine ; Kelly Ruggles in the Institute of Systems Genetics, Paolo Cotzia, in the Department of Pathology; Amy Rapkiewicz, adjunct associate professor, Department of Pathology at NYU Langone Long Island Hospital; and Hannah Burgess, Ian Mohr, and Kenneth Stapleford in the Department of Microbiology. Also authors were Alexander Fields, Lucy Kornblith, and Stefania Pittaluga of the Department of Surgery at the University of California, San Francisco; Grace Smith of the Center for Cancer Research at the National Cancer Institute, Matthew Neal of the Department of Surgery at the University of Pittsburgh, and Deepak Voora of the Duke Center for Applied Genomics & Precision Medicine at Duke University School of Medicine.
The study was funded by National Institutes of Health grants 1OT2HL156812-01, R35HL144993, R35GM119526, R01HL118049, 1T32GM136573-01, UL1TR001445, and P30CA016087; American Heart Association grant 18CDA34110203AHA, American Society of Hematology grant 18-A0-00-1001884, Deutsche Forschungsgemeinschaft (German Research Foundation) grant RO 6121/1-1; as well as through funding from the National Center for Advancing Translational Sciences (NCATS).










