Antibody Binding-Site Conserved Across COVID-19 Virus Variants – Big Implications for Future Vaccines
0 View
Share this Video
- Publish Date:
- 16 May, 2021
- Category:
- Covid
- Video License
- Standard License
- Imported From:
- Youtube
Tags
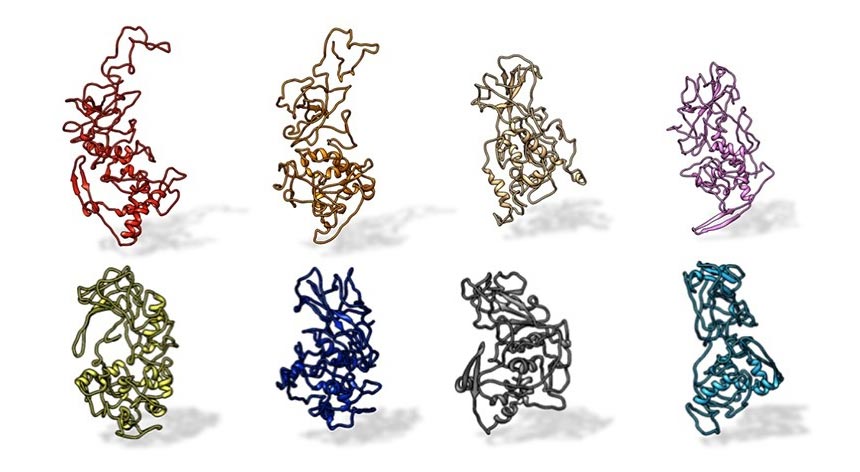
A Penn State research team found that the N protein on SARS-CoV-2 is preserved in all SARS-related pandemic coronaviruses (top, from left: SARS-CoV-2, civet, SARS-CoV, MERS). The protein differs from other coronaviruses, such as those that cause the common cold (bottom, from left: OC43, HKU1, NL63 and 229E). Credit: Kelly Lab / Penn State
The structural disclosure could have implications as a therapeutic target in all SARS-CoV-2 variants.
A small protein from SARS-CoV-2, the coronavirus that causes COVID-19, could have major implications for future treatments, according to a team of Penn State researchers.
Using a new toolkit of approaches, the scientists discovered the first full structure of the Nucleocapsid (N) protein and how antibodies from COVID-19 patients interact with that protein. They also found that the structure appears similar in many coronaviruses, including recent COVID-19 variants, making it an ideal target for advanced treatments and vaccines. They reported their results on a nanoscale.
“We discovered new features about the N protein structure that could have major implications in antibody testing and the long-term effects of all SARS-related pandemic viruses,” said Deb Kelly, professor of biomedical engineering (BME), Huck Chair in Molecular Biophysics and director. from the Penn State Center for Structural Oncology, who led the study. Since it appears that the N protein is conserved in the variants of SARS-CoV-2 and SARS-CoV-1, therapies designed to target the N protein may help eliminate the heavier or persistent symptoms that some people experience. “
Most of the diagnostic tests and vaccines available for COVID-19 are designed based on a larger SARS-CoV-2 protein – the Spike protein – where the virus attaches to healthy cells to start the invasion process.
The Pfizer / BioNTech and Moderna vaccines are designed to help recipients produce antibodies that protect against the Spike protein. However, Kelly said the Spike protein can mutate easily, resulting in the variants that have emerged in the United Kingdom, South Africa, Brazil, and the United States.
Unlike the outer Spike protein, the N protein is encapsulated in the virus, protected from environmental factors that cause the Spike protein to change. However, in the blood, the N protein floats freely after it is released from infected cells. The free-roaming protein triggers a strong immune response, leading to the production of protective antibodies. Most antibody test kits look for the N protein to determine if a person has been previously infected with the virus – unlike diagnostic tests that look for the Spike protein to determine if a person is currently infected.
“Everyone is looking at the Spike protein and fewer studies are being conducted on the N protein,” said Michael Casasanta, lead author of the paper and postdoctoral researcher in the Kelly laboratory. There was a hole. We saw an opportunity – we had the ideas and the resources to see what the N protein looks like. “
Initially, the researchers examined the N protein sequences of humans, as well as several animals believed to be potential sources of the pandemic, such as bats, civets and pangolins. According to Casasanta, they all looked alike, but clearly different.
“The sequences can predict the structure of any of these N proteins, but you can’t get all of the information from a prediction – you have to see the actual 3D structure,” Casasanta said. “We’ve converged the technology to see something new in a new way.”
The researchers used an electron microscope to image both the N protein and the site on the N protein where antibodies bind, using serum from COVID-19 patients, and developed a 3D computer model of the structure. They found that the antibody binding site remained the same in each sample, making it a potential target for treating humans with one of the well-known COVID-19 variants.
“If a therapeutic agent can be designed to target the N protein binding site, it may help reduce inflammation and other persistent immune responses to COVID-19, especially in COVID long-haul vehicles,” Kelly said, referring to people taking COVID-19 experiencing symptoms. for six weeks or longer.
The team purchased purified N proteins, meaning the samples contained only N proteins, from RayBiotech Life and applied them to microchips developed in collaboration with Protochips Inc. The microchips are made of silicon nitride, as opposed to a more traditional porous carbon, and they contain thin wells with special coatings that pull the N proteins to their surface. Once prepared, the samples were flash frozen and examined by cryoelectron microscopy.
Kelly thanked Penn State’s unique combination of microchips, thinner ice samples and advanced electron microscopes, which are equipped with state-of-the-art detectors, modified by the Direct Electron company, for delivering the highest resolution visualization of SARS lightweight molecules. -CoV-2 so far.
“The combination of the technology resulted in a unique discovery,” said Kelly. “It used to be like trying to look at something frozen in the middle of the lake. Now we look at it through an ice cube. We can see smaller entities with much more detail and higher accuracy. “
Reference: “Microchip-Based Structure Determination of Low Molecular Weight Proteins Using Cryoelectron Microscopy” by Michael A. Casasanta, GM Jonaid, Liam Kaylor, William Y. Luqiu, Maria J. Solares, Mariah L. Schroen, William J. Dearnaley, Jarad Wilson, Madeline J. Dukes, and Deborah F. Kelly, Apr 1, 2021, Nanoscale.
DOI: 10.1039 / D1NR00388G
Casasanta and Kelly are also affiliated with Penn State’s Materials Research Institute (MRI). Co-authors include GM Jonaid, BME and Bioinformatics and Genomics Graduate Program at Penn State’s Huck Institutes of the Life Sciences; Liam Kaylor and Maria J. Solares, BME and graduate program in molecular, cellular and integrative life sciences from the Huck Institutes of the Life Sciences; William Y. Luqiu, MRI and Electrical and Computer Engineering Department at Duke University; Mariah Schroen, MRI; William J. Dearnaley, BME and MRI; Jared Wilson, RayBiotech Life; and Madeline J. Dukes, Protochips Inc.
The National Cancer Institute of the National Institutes of Health and the Center for Structural Oncology at Penn State’s Huck Institutes of the Life Sciences funded this work.










